 Ready to test your knowledge of acids and bases with a few practice problems?
Ready to test your knowledge of acids and bases with a few practice problems?
This quiz is designed to follow my Acids and Bases tutorial video series. But I'm not looking to see if you simply ‘memorized' the concepts. The videos use simple examples to demonstrate concepts, these questions will test you at a slightly higher level.
So give it a shot, see how you do, then download the solutions at the end of the quiz.
Be sure to grab the Acid/Base Cheat Sheet to help you along.
Acid Base Practice Question 1
Which is the stronger acid?
Acid Base Practice Question 2
Rank the following ionic compounds in order of increasing base strength.
Acid Base Practice Question 3
Which of the following is the stronger base? (Not asking if it forms a stronger conjugate base)
Update: Although many organic textbooks state the pKa of water to be 15.7, the correct value for the pKa of water is 14.e
Acid Base Practice Question 4
Which of the following is the stronger acid?
Acid Base Practice Question 5
Which of the following forms a stronger acid upon reacting with a proton?
Acid Base Practice Question 6
Rank the following in order of increasing base strength
Acid Base Practice Question 7
Rank the following in order of increasing acidity.
Acid Base Practice Question 8
Rank the following in order of increasing acidity.
Acid Base Practice Question 9
Label the acid, base, conjugate acid and conjugate base in this reaction
Hint: Not sure which is which? Watch Acid/base video 1 for a quick refresher
Acid Base Practice Question 10
Label the acid, base, conjugate acid and conjugate base in this reaction
Hint: Not sure which is which? Watch Acid/base video 1 for a quick refresher
Acid Base Practice Question 11
Rank the following in order of decreasing acidity
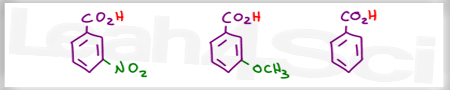
Acid Base Practice Question 12
Rank the following in order of increasing acidity
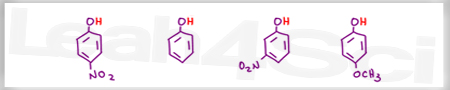
Acid Base Practice Question 13
Complete this acid base reaction and determine the position of equilibrium
Acid Base Practice Question 14
Complete this acid base reaction and determine the position of equilibrium
Acid Base Practice Question 15
Which of the following forms a weaker conjugate base?
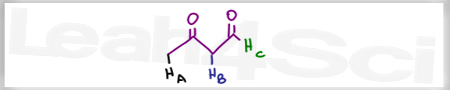
Acid Base Practice Question 16
Which of the the labeled protons is more acidic, and why?
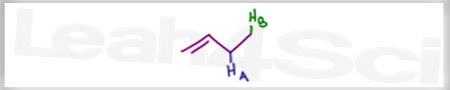
Acid Base Practice Question 17
Rank the labeled protons in order of increasing acidity.
Acid Base Practice Question 18
Which is the stronger acid?
Acid Base Practice Question 19
Which is the stronger acid and why?
Acid Base Practice Question 20
Which forms a weaker conjugate base and why?
Acid Base Bonus Practice Question 1
Rank the following in order of increasing acidity. Use resonance to back up your explanation.
Acid Base Bonus Practice Question 2
Which is the stronger acid and why?
Ready to see how you did?
Don't just right to t he solutions. Instead challenge yourself to try every question first.
Send Me The Solutions!










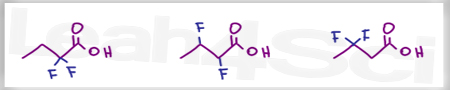










I believe the answer to Question 4 is incorrect. The answer key says “CH3OH > HF”, however since the Ka of HF is larger than the Ka of CH3OH, that would make HF a stronger acid. I believe that’s what the comments from 3 years ago were trying to address.
please can you tell me the link of the video in which you have explained the solution of bonus question 1
I think that there’s a mistake in solutions of question 12 as m-nitrobenzoic acid is obviously more acidic than benzoic acid due to strong -I inductive effect of the nitrate group. I think you should check that.
Hello, I have a question about problem #4. In the solutions, it says CH3OH is a stronger acid than HF. However, the higher the Ka, the lower the pKa, and the stronger the acid. It shows that HF has a higher Ka than CH3OH so shouldn’t it be the stronger acid? Thank you.
Hi! Thank you for the problems and solutions. Question 4 have me confused a bit. Larger Ka value means strong acid, but in your solution you wrote CH3OH > HF
I have not received pdf solutions.Also checked in spam .Can you please email me!!.Thank you!
Hi Leah:
I’m confused by the answer to questions 14. The products (compounds to the right of arrow) are neutral whereas the reactants on the left side of the arrow are charged. Therefore, wouldn’t that supersede the resonance and electronegativity that stabilizes the charged reactants? I assumed the arrow would point to the right.
Thank you!
I think you should check out some of your solutions: in problem 12 I think the -OCH3 benzene would be the strongest base/weakest acid due to the electron donating tendencies of the -OCH3 group.
I have an exam coming up and your videos as well as this quiz really helped me make sense of some concepts I have been having trouble with. Thank you!
You’re Welcome, Sarah!
I have done to write all problems so easily thanks lovely girl
Plz send me your email id.
Hello!
I have not received the PDF solutions for this quiz. I’m not sure if I am doing something wrong but I have tried numerous times.
Please help!
Have you checked your spam folder? You may try a different email if it still doesn’t come through.
please our professor gave us primary , secondary and tertiary amines and asked us to compare between them in an increasing acidity order
Alkyl groups make amines more basic
Hello Liah I haven’t received any email for the solutions despite clicking on the link and giving required information. Can u mail it to me or give me ur email address?
Lakshita: Email me
I can’t find the quiz solutions. Where are they?
They’re linked at the bottom of the page. Email me if you still can’t find it
Hey Leah, the email won’t send for the acid and base solutions of the quiz. If you can email it to me that would be great!
Thanks!
Email me
i cant find the solutions to the quizzes
Mariam: it’s linked at the bottom of the page
To everyone asking about the m-nitrobenzoic acid: m-nitrobenzoic acid is not a weaker acid than benzoic acid. This is an error in her solutions. m-Nitrobenzoic acid is a stronger acid than benzoic acid. The pKa of m-nitrobenzoic acid is 3.49 while that of benzoic acid is 4.20. This is due to the inductive effect of the electron withdrawing nitro group stabilizing the conjugate base.
Hello, I have a question on number 4.I understand how the higher the Ka, the lower the pKa, and so the stronger the acid. But, the Ka of HF is higher (^-4) than the Ka of CH3OH (^-16), meaning that the pKa of HF will come out larger than CH3OH and so becomes the stronger acid. But, the answer is CH3OH. May you explain this please, thank you.
High Ka or lower pKa is the stronger acid making HF stronger
On the solution document, I think there is a mistake on question 4. The HF should be the stronger acid, so the symbol should be flipped.
Yes, i did but i stil confused. Because I think that Nitrogen (with the (+) charge) which can pull the electrons towards it, and make the conjugate base be more stable.
That’s correct, so I guess I don’t understand your question
Because I saw on your quiz solution you said that m-nitrobenzoic acid is the weakest while we compare the acid strength among m-Nitrobenzoic acid, benzoic acid and m-methyl benzoate.
Can you explain to me. Why m-nitrobenzoic acid is weaker than benzoic acid. Thanks alot
Did you check the quiz solutions?
yes leah that one has me confused as well.