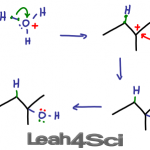
Acid catalyzed hydration of alkenes involves replacing the pi bond on an alkene with a water molecule. This is done by adding an alcohol to the more substituted carbon atom, and hydrogen to the less substituted carbon atom. This reaction follows Markovnikov’s rule and may undergo a carbocation rearrangement. If it is chiral, the product […]
Backpack Trick for Organic Chemistry Reactions
When it comes to organic chemistry reactions, many students focus on learning the EXACT steps by memorizing the SPECIFIC molecules presented in their books and lecture slides. Then, when something looks even slightly different on the exam, they panic! One such scenario: replacing water with alcohol in a reaction sequence – either as the attacking molecule or […]
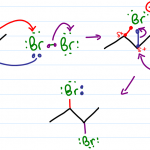
Orgo Mechanisms with Common Arrow Patterns
Organic chemistry is all about mechanisms. And while the sheer volume may feel overwhelming, they all boil down to a few simple types of mechanism steps. In Part 1 and Part 2 we looked at the four common mechanism types: Nucleophilic attack, Loss of Leaving Group, Proton transfer and Rearrangements. In this video we’ll look at TWO […]
Intro to Orgo Mechanisms Nucleophilic Attack and Loss of Leaving Group
Organic chemistry is all about the reaction mechanisms and moving arrows. Video 1 in this Mechanism series will help you understand what the heck these mechanisms are all about. We’ll start with a quick overview of what to look for, how to write the mechanisms, reaction arrows, and more. Then we’ll look at the first […]
Proton Transfer and Rearrangement Mechanisms in Organic Chemistry
Proton transfer and rearrangements are 2 more common mechanism patterns you will face in organic chemistry. This is in addition to Nucleophilic attack and Loss of Leaving Group discussed in Mechanism Video Part 1. You’ll see multiple proton transfer examples in action including turning a bad leaving group into a good leaving group! Rearrangement mechanism are broken down for […]
A Simple Approach to Retrosynthesis in Organic Chemistry
In Organic Chemistry, synthesis and retrosynthesis go hand in hand. While there isn’t a clear distinction, I like to think of synthesis as forward thinking and retrosynthesis as the reverse. Synthesis is a topic that is typically introduced in Organic Chemistry 1, right after studying alkyne reactions. You’ll be utilizing it again and again as […]
Halogenation of Alkenes – Organic Chemistry Reaction Mechanism
Reaction Overview: The alkene halogenation reaction, specifically bromination or chlorination, is one in which a dihalide such as Cl2 or Br2 is added to a molecule after breaking the carbon to carbon double bond. The halides add to neighboring carbons from opposite faces of the molecule. The resulting product is a vicinal (neighboring) dihalide. Summary of […]
Resonance Structures
Orgo Tutorial Videos
Orgo tutorial videos on YouTube