Oxymercuration-Demercuration, also referred to as oxymercuration-reduction is slightly more confusing than that average alkene reaction. I think it's a combination of the extra steps, involvement of mercury, and the mysterious reduction by sodium borohydride. But don't let this confuse you.
The video below takes you through the entire reaction mechanism with step by step explanations of every moving arrow, regioselectivity, and reason for lack of carbocation rearrangement.
Watch this video on YouTube or read the transcript (coming soon)
Watch the previous Video: Ring Expansion via Methyl and Hydride Shift
Watch Next video: Alkoxymercuration-demercuration
Return to: Alkene Reaction Mechanism home page;
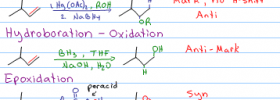


What is the mechanism for Demercuration? I mean how Hg(OAC) gets replaced by hydrogen.
will this be anti addition?
These videos are very helpful! It would be helpful if you showed which steps are reversible vs. non reversible.
Nice explanation but shouldn’t H2O attack the carbon with more partial positive charge (better electropile/electron deficient region) i.e. 1 degree terminal C ?
why wouldn’t the h2o form a bond with the mercury instead of attacking a carbon that’s only partially positive? hg has a positive charge so to me it makes more sense that the h2o would attack the hg than the carbon.
You helped me get an A in orgo 1 first time taking it! I think I watched every single one of your videos, thank you so much Leah!
The reaction is always a “Syn-Addition”?
At the end of step 2 do you still have acetic acid as a product? Or is that somehow used up during the reduction mechanism?
Acetate/acedic acid is a side product
how can mercury have a pair of electrons? i thought its electron configuration was [xe]4f14 5d10 6s2
This is a tricky concept Nick and I think the reason most professors skip it. Don’t forget that d-shell electrons are high in energy and may be called upon to react at any point in the mechanism.
How can one compound be attached to two carbon atoms at same time ? Here HgOAc is attached to both primary and secondary?
One bond is not dependent on the second. Hg has the ability to form multiple bonds
Oh k thank you….. I love the way u teach
Thank you Latha
THANK YOU! I am in OChem 1 and have been struggling through the alkene reactions. You explain this so simply and give fantastic explanations for WHY the intermediates like HgOAc form the two bonds in the molecule. Thank you again!!
Thanks Jess
I cant play the video kindly send m link to the video
This is a YouTube video, if it doesn’t work for you check your browser settings or try another browser
You are my new favorite teacher! videos are clear and informative.
Thank you David. Are you in Orgo 1 or Orgo 2?