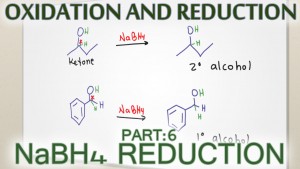
 Sodium Borohydride NaBH4 is a common reducing reagent used with carbonyl compounds. NaBH4 is a weak reducing agent and will only reduce ketones and aldehyes. Watch for the explanation below including reaction, mechanism, and practice problems.
Sodium Borohydride NaBH4 is a common reducing reagent used with carbonyl compounds. NaBH4 is a weak reducing agent and will only reduce ketones and aldehyes. Watch for the explanation below including reaction, mechanism, and practice problems.
(Watch on YouTube: NaBH4 Reduction. Click cc on the bottom right for video transcript)



Excellent explanation May God strengthen your knowledge…