Acid catalyzed hydration of alkenes involves replacing the pi bond on an alkene with a water molecule.
This is done by adding an alcohol to the more substituted carbon atom, and hydrogen to the less substituted carbon atom.
This reaction follows Markovnikov’s rule and may undergo a carbocation rearrangement. If it is chiral, the product will be racemic due to the carbocation intermediate.

Summary of the Acid Catalyzed Hydration Mechanism
- Nucleophilic pi bond attacks a proton in solution, pi bond breaks in the process
- Carbocation forms on the more substituted carbon (less substituted carbon has the H)
- Water molecule from solution uses oxygen electrons to attack the carbocation forming oxonium (see below)
- Another water molecule in solution removes the extra proton
- Product: an alcohol with hydroxyl group on the more substituted carbon
Mechanism Overview and Step By Step Explanation

Want to see more? Watch my Acid Catalyzed Hydration Video Tutorial HERE
Key Reaction Notes
- The acid catalyst dissociates to give H+ in solution — H+ can be shown alone but is typically attached to water to form an H3O+ Hydronium ion.
- Be on lookout for carbocation rearrangement due to carbocation intermediate.
- Carbocation intermediate is sp2 hybridized. This allows water to attack top or bottom for racemic product.
- This is a solvolysis reaction where the solvent (water) partakes in the reaction. Apply the backpack trick if solvent = alcohol.
- Despite multiple oxonium appearances in this reaction (oxygen with +1 formal charge), it's never the positive oxygen that gets attacked. When oxygen is bound to Hydrogen the oxygen pulls on the electrons to make itself slightly less positive. This leaves the H proton partially exposed and partially positive resulting in H rather than O getting attacked.
Acid catalyzed hydration is an important reaction in your orgo synthesis arsenal.
In addition to this reaction, alkenes can also be converted to alcohols using Oxymercuration-Demercuration or Hydroboration Oxidation.
While the alcohol functional group is the same, it’s the regioselectivity and stereospecificity that sets each reaction apart. This is discussed in Alkene To Alcohol Which Reaction To Use (Coming Soon).
What is Happening in This Reaction
Activating the Acid Catalyst
When using a strong acid, the acid dissociation in solution which forms hydronium is automatic and often taken for granted. This is the source of confusion for students who simply memorize steps.
According to the Arrhenius and Bronsted-Lowry definitions, an acid is one that dissociates to give an H+ in solution.
Regardless of your starting acid, if it is strong enough to dissociate, the acid IN SOLUTION is merely an H+. — This is why many problems will show catalytic acid (cat acid) or simply H+ for the reaction.

Remember that protons don’t float around freely in solution. Instead they are picked up by a solvent molecule, in this case water, to form a protonated solvent molecule.
When the solvent is water the product is hydronium.

Some professors may ask you to show the mechanism for the catalyst, as shown below for sulfuric acid dissolving in water.
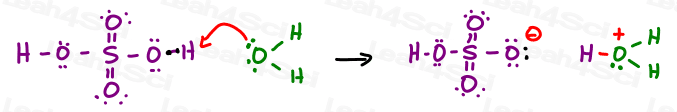
Others will simply allow you to assume that an acid catalyst is the source of H+ in solution.
In this case simply cross out the catalyst and write H+ to avoid any confusion.
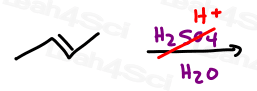
Attack of the Pi Bond
As with any reaction you want to ask a series of questions to guide you.
What is most positive in solution acting as the electrophile?
The proton or hydronium.
What is most negative in solution acting as the nucleophile?
The alkene pi bond.
And so the reaction begins with the nucleophilic pi electrons reaching for a proton in solution, or one sitting on hydronium.
Since hydrogen can only have 1 bond, when it gets attacked it must let go of the electrons binding it to the water molecule.
Carbocation Formation
Following Markovnikov’s Rule, the proton adds to the more substituted carbon atom leaving the less substituted (former pi bound) carbon deficient.
With only 3 bonds and an empty p orbital = a carbocation forms.

Quickly verify using the Formal Charge Shortcut.
Look out for a potential carbocation rearrangement, most notably when a secondary carbocation is adjacent to a tertiary or quaternary carbon.

Now that the carbocation is most positive in solution (the new electrophile), and will attract the attention of a nearby water molecule, particularly the partially negative oxygen atom with its lone pair of electrons.
Oxygen uses its electrons to attack the carbocation. This forms a bond between itself and carbon.

This gives oxygen a total of 3 bonds and 1 lone pair for a formal charge of +1.
The positive oxygen or oxonium is unhappy and starts pulling on the electrons between itself and one of the hydrogen atoms.
The more oxygen pulls on hydrogen, the less ‘positive’ the oxygen, but the more positive the hydrogen atom.
THIS is why hydrogen gets attacked even when it’s the oxygen atom that has a positive charge.
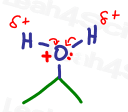
Remember, this reaction takes place dissolved IN water. This means that you have billions and billions of water molecules available, ready to step in and participate in the reaction!
Proton Transfer to Form the Alcohol
The final step of this reaction is the removal of the extra H atom to yield a neutral alcohol.
Some professors will show the conjugate base of the initial acid catalyst pulling the proton.
Others will show a solvent molecule pulling the proton.
Still others will show B for ‘base’ implying that ANYTHING can pull off the final proton. 
Unless your professor is picky, it REALLY doesn’t matter. The end product is the same.
I tend to show the solvent molecule because if you look at the ratio of catalyst to solvent
you have millions more solvent molecules compared to the catalyst.
When it comes to reactions it’s simply a numbers game.
Which partially negative molecule is available nearby to deprotonate the oxygen?
This molecule will grab the proton.
Stereochemistry of the Final Product
This reaction has the potential to form an optically active product.
Look at the carbon atom holding the alcohol.
Does it contain 4 unique groups?
If not, your product is achiral.

If yes, your product will be chiral but racemic.
Recall that a racemic product is one that randomly forms R or S products so that the ratio is approximately 50/50.

Why is the final alcohol racemic?
The carbocation intermediate is sp2 hybridized.
The molecular geometry of an sp2 carbon is trigonal planar or simply put, flat.
The incoming oxygen can attack the flat carbocation with nearly equal probability from the top or bottom — I say ‘nearly’ equal because there may be steric considerations but that is beyond the scope of undergraduate organic chemistry.
In the above example, When oxygen attacks from the top, one type of chiral molecule will form.
When water attacks from the top, the final alcohol has R stereochemistry.
When it attacks from the bottom the enantiomer will form as demonstrated in the drawing below.
When water attacks from the bottom, the final product will have S stereochemistry.
Confused? Find out more in my Chirality and Stereochemistry Video Tutorial Series.
Acid Catalyzed Hydration in Alcohol
As with any solvolysis reaction, this reaction can be carried out in an alcohol solvent instead of water to yield an entirely different product: An ether!
The reaction mechanism is exactly the same, the only difference is that the additional -R group on the alcohol gives an -OR instead of -OH as the final product.
The simplest way to learn and practice this reaction in alcohol is by utilizing the Backpack Trick.

This tutorial is just the first step to learning and understand organic chemistry concepts and reactions. Don’t let the semester take you by surprise forcing you to play catch-up. Click below to download EVERY Leah4sci Orgo Cheat Sheet in a single full color PDF download. You’ll also get access to tutorials, study tips and tricks, and Upcoming LIVE Workshop announcements!
Want to see more? Watch my Acid Catalyzed Hydration Video Tutorial HERE
Also, test your understanding with my FREE Alkene Reaction Practice Quiz


Leave a Reply