 Below is the transcript of my tutorial video in Ionic, Polar Covalent and Non-Polar Covalent Bonding.
Below is the transcript of my tutorial video in Ionic, Polar Covalent and Non-Polar Covalent Bonding.
(Click here to watch on YouTube)
[Start Transcript]
Leah here from leah4sci.com and in this Orgo basics video we’ll talk about bonding as it’ll show up in your Organic Chemistry course including Ionic Bonding, Polar covalent and non polar covalent. When you cover this in General Chemistry you look at the idea of the octet rule and how atoms that do not have a complete octet will interact with other atoms to help each other reach that full valence shell of electrons.

Let’s start with Ionic Bonding. An Ion is an atom or molecule that gained or lost an electron and since the number of protons and electrons are not equal to each other we’ll have a net negative or net positive charge. This is easy to see in atoms like Sodium which has one valence electrons and when lost gets a net charge of plus 1 or Chlorine which has 7 valence electrons and when it gains one more gets a net negative charge.
In General Chemistry you learn how to calculate the difference in electronegativity and to recognize when the difference is greater than two, you’ll have an ionic bond or have one atom steal from another. In Organic Chemistry it’s more a concept of recognizing when these bonds take place.

If you look at the periodic table, you should be able to recognize the atoms that are likely to form a covalent bond based on the number of valence electrons and where they’re located. I go through the idea of Periodic Table and Trends In another video which you can find on my website at https://leah4sci.com/OrganicChemistry.
Looking at the table, in group 1 all the elements have one valence electron. Since the quickest way to reach a full octet is to give away that valence electron. You will find group 1 elements often forming a cation or positive ion. You’ll typically find the group 1 elements at the start of a larger molecule written out as a neutral compound but you should recognize them as a cation and therefore whatever follows is likely an anion.
For example, anytime you see Lithium, Sodium or Potassium followed by something that has an Oxygen or Nitrogen we’re likely looking at an ionic compound. Common examples include something like NaOCH3 which is a positive Sodium spectator and Methoxide is your anion. You will also see something like Na NH2 which ones again has Sodium as our positive spectator ion and NH2 minus as our negative Ion.
Sodium as the positive spectator ion and NH2 minus as the negative and reactive Anion. This is very important to recognize because in Organic Chemistry reactions you have to be able to look at a reagent, look at the chemical and determine if it’s strong or weak and that negative charge will be a big indicator on what’s to follow.

Moving to the right of the table, for the most part, the elements in the middle of the Periodic Table don’t show up as frequently in Organic Chemistry but if they do, since they are metals they tend to be positive or partially positive. Moving to the right in the Periodic Table we have group 8 which is the noble gasses.
Since they already have a complete Octet, they’re not interested in interacting with other atoms and you’ll seldom find them either in bonds or in ionic compounds. But in group 7 where we’re 1 electron away from a complete octet once again we’re going to see Ionic or very close to ionic compounds. We already know that the halogens Flourine, Chlorine, Bromine and Iodine need one more electron to complete their octet forming a minus 1 ion. In Organic Chemistry will show up as both covalently bound which we’ll talk about shortly and Ionically bound.
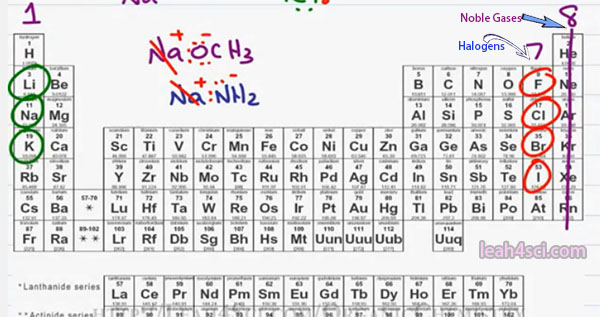
Moving slightly to the left, there are three more atoms that you want to recognize, Nitrogen, Oxygen and Sulfur will open show up in reactions and sometimes negative. For example in the Sodium methoxide we showed here, Oxygen is bound covalently to the carbon. It has a complete octet but it has more that it should have giving us the negative charge, something we’ll talk about in a later video when we discuss formal charge.
Same thing with Nitrogen, notice that it has extra electrons and even though it’s bound to hydrogen it still Ionic and therefore still has that negative charge.
Covalent bonds occur when you have an atom sharing its electrons instead of stealing or giving away electrons in order to complete its octet. The most common example of this especially to Organic Chemistry is the carbon atom. Carbon has a total of 4 valence electrons and to complete its octet ionically it would have to give away 4 or gain 4 both of which are too extreme and too impossible so you’ll often find carbon bound 4 times to different atoms sometimes overlapping atoms but this way in sharing its electrons with another atom it can complete its octet.
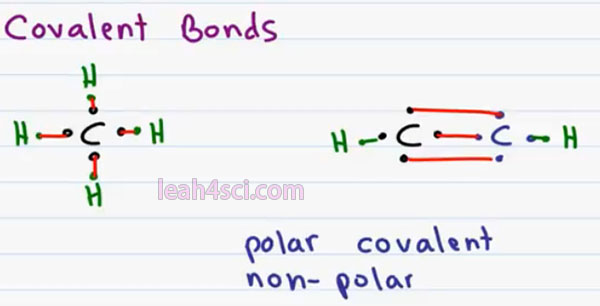
For example in the molecule Methane, carbon is bound to 4 hydrogen atoms so we have Carbon’s 4 valence electros plus 1 each from the four hydrogen atoms giving us a complete Octet of 8. Hydrogen is an exception to the rule so its complete octet is only 2. But carbon can also bind more than once to different atoms. For example if we bind carbon to another carbon three times in a triple bond and then add hydrogens at the end, each carbon still has a total of 8 electrons because every bond counts for two.
But in this case because we shared 3 bonds between the black and blue carbon atoms it’s considered a triple bond. Within covalent bonding we have two types; Polar Covalent and Non-Polar Covalent.
In General Chemistry you learn how to take the difference in electronegativity to determine when the bond is Polar and when it’s Non-Polar. In Organic Chemistry once again you have to understand the nature of these bonds simply by knowing the atoms involve and where they show up in the Periodic Table.
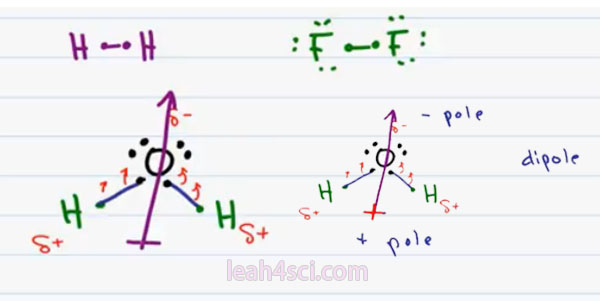
We’ll start with Non-Polar because it’s simpler. If the two atoms are very close in electronegativity, for example a hydrogen bound to another hydrogen or a Flourine bound to another Flourine. Because the difference in electronegativity is the same, no atom is holding onto the electrons more than the other and this results in a non polar bond.
Notice that I specifically chose a low electronegativity Atom such as Hydrogen and a high electronegativity such as Flourine because the weak hydrogen is competing with the weak hydrogen and the strong Flourine is competing with a strong Flourine.
So now let’s move on to Polar Covalent. When you have atoms that are not as equally matched in terms of their electronegativity, even though there are some sharing between the atoms, it will not be equal and the net charge even though zero won’t be perfectly centered. We’ll use water as our example. Oxygen has 6 valence electrons and needs 2 more to complete its Octet. Hydrogen atoms each have one electron and need one more to complete their Octet.
And so we’ll create a covalent bond between Oxygen and Hydrogen completing each atom’s octet. But what you want to keep in mind is that, even though they’re sharing, Oxygen is more electronegative and is not as willing to share its electrons compared to Hydrogen. I want you to picture this scenario; say you have a younger sibling who’s just a couple years younger than you. You have a relative that comes from out of town and gives to the two of you an amazing toy to share. When you have adults in the room you have to share because you don’t have a choice. As soon as the adults leave, given you’re the older one and we’re assuming that you bigger and stronger you’re gonna hug that toy and start playing with it by yourself.
If an adult peeks into the room and ask, hey kids are you sharing, you’re like yeah of course we’re sharing, you kinda push it over to your sibling but when the adults are not around, even though you’re sharing, you’re taking advantage of your strength and your superior age to play with that toy more because let’s face it, you both want it and you’re bigger and stronger and you win.
Oxygen is the same way. Oxygen’s high electronegativity makes it want those electrons more. So even though it’s sharing with Hydrogen it tends to pull on the electrons towards itself almost hugging the electrons. In that fraction of a second in time when the electrons are closer to Oxygen, we have all that concentrated negativity making the Oxygen partially negative. Hydrogen which is really just a proton and a neutron weakly neutral, but when the electrons are being pulled away from it, the nucleus’ slightly exposed giving every hydrogen a partial positive charge. The Delta symbol implies “partial charge” so we don’t have a complete charge.
The net molecule is still neutral but Oxygen is partially negative and hydrogen is partially positive. Notice the way that this is drawn, we have all the negativity concentrated at the top and the positivity is slightly to the right and left which cancels out, that means the positivity is concentrated at the bottom.
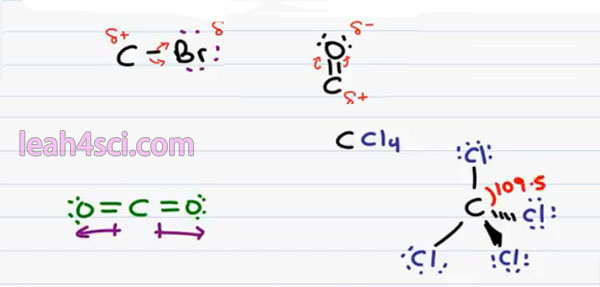
If we were to draw a vector going from positive to negative, the Vector or dipole moment would go upward towards the Oxygen. The way to remember which is the negative and positive end, envision a positive on the tail of this dipole moment pointing towards the negative. So we can consider the bottom as the positive pole and the top as the negative pole. And having a positive and a negative gives us two pole or a dipole.
And the type of covalent bond that forms this dipole is considered a Polar Covalent Bond. This is very important in Organic Chemistry reactions because neutral atoms will not react to strongly as polar are charged atoms. So for example, if you have a Carbon bound to a Bromine, you should recognize Bromine as an electronegative halogen pulling on the bond between itself and carbon giving it a partial negative charge making the carbon partially positive and therefore susceptible to an attack.
Later on in your course, you’ll come across the carbonyl which is a carbon double bound to an Oxygen atom. Here, not only do we have a more electronegative atom bound to carbon, but there are two bonds, that means there’s twice as many electron for oxygen to attempt to hug and once again when Oxygen hugs these electrons we can have a partial negative carbonyl Oxygen and a partial positive carbonyl carbon.
There’s one more thing I want to discuss about Polarity as it relates to Organic Chemistry. Sometimes you’ll see a molecule but even though the individual bonds are polar, the overall molecule will not be Polar the direction in which the polar atoms are pulling are going to cancel out. A common example is the molecule carbon dioxide. We have carbon double bound to an oxygen atom which we’ve already proven as Polar but it’s also double bound to another Oxygen atom on the opposite side.
If we draw an arrow from the positive to negative pole where the dipole moment, we have to draw one to the right and one to the left. But notice that they perfectly cancel out giving me a net polarity of zero and therefore a non-polar molecule. This one is somewhat obvious. A little less obvious is a Tetrahedral Atom.
For example, if I have carbon bound to four Chlorine atoms CCl4. If we draw this out in three dimensions the way you have to in Organic Chemistry, we have 2 bonds in the plane, 1 down and out off the page and down and into the page. Every bond is between a Carbon and a Chlorine atom and every bond is individually Polar because Chlorine is more electronegative than carbon & pulls the electron ends to be away from carbon.
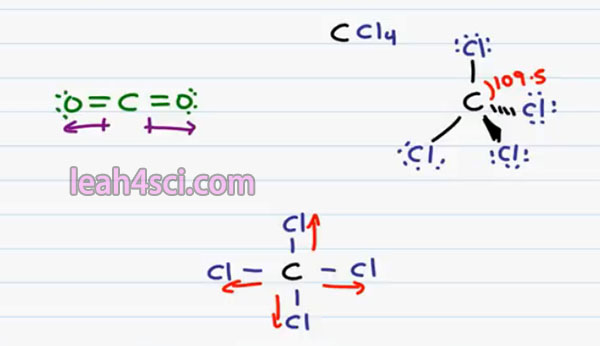
But keep in mind that in a tetrahedron, we have 4 equidistant spaced atoms with the bond angle between them all of 109.5. That means that even though Chlorine to Carbon is Polar, all the polarity is canceled out because they’re being pulled in completely opposite directions.
This is a little easier to see if you draw this molecule in two dimensions. This is not correct for Organic Chemistry but it does help you see what’s going on. Recognize that if every Chlorine pulls away from carbon the net pull on this molecule’s cancel out giving us a Non-Polar Molecule. This is something you’ll see when you’re dealing with solvents. This is a non-polar or inert solvent.
Be sure to join me in the next video where we talk about hybridization, bond angles and geometry as it relates to Organic Chemistry. You can find that video along with this entire series by visiting my website at https://leah4sci.com/OrgoBasics.
Are you struggling with Organic Chemistry? Are you looking for resources and information to guide you through the course and help you succeed? If so, then I have a deal for you. A free copy of my ebook “10 Secrets to Acing Organic Chemistry”. Use the link below or visit orgosecrets.com to grab your free copy. After downloading your free copy of my ebook, you’ll begin receiving my exclusive email updates with cheat sheets, reaction guides, study tips and so much more. You’ll also be the first to know when I have a new video or live review coming up.
If you enjoyed this video please click the thumbs up and share with your organic chemistry friends and classmates. I will be uploading many videos over the course of the semester so if you haven’t subscribed to my channel yet, do so right now to be sure that you don’t miss out.
[End Transcript]
Watch The Video Here: Ionic, Polar Covalent and Non-Polar Covalent Bonding in Organic Chemistry


Leave a Reply