 Substitution and Elimination reactions are potentially the most difficult concepts covered at the Organic Chemistry 1 level. In addition to studying the SN1 SN2 E1 and E2 reaction mechanisms, you also have to understand the similarities and differences so that you can derive the correct products for specific reaction conditions.
Substitution and Elimination reactions are potentially the most difficult concepts covered at the Organic Chemistry 1 level. In addition to studying the SN1 SN2 E1 and E2 reaction mechanisms, you also have to understand the similarities and differences so that you can derive the correct products for specific reaction conditions.
I've put together this medium/tricky quiz to help you practice the reactions and gauge your level of comprehension.
Not fully confident yet? Go back and review my entire SN1 SN2 E1 E2 reaction video series and study the associated cheat sheet before getting started. So take your time as you go through the questions. If you get stuck just visit the video series page (linked above) and watch the related tutorial.
Download PDF Solutions posted at the end of the quiz.
Substitution Elimination Practice Problems
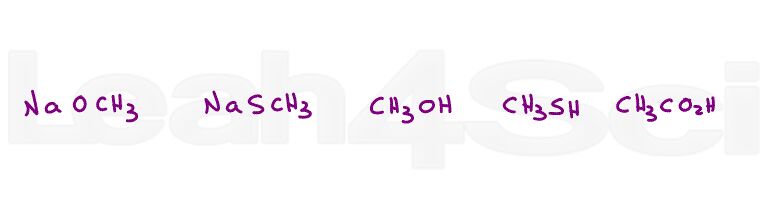
Question 1: Rank the following nucleophiles in order of increasing strength
Need help? Watch the Nucleophile vs Base videos in the series
Question 2: Arrange the following molecules in order of increasing reactivity/ability for a unimolecular elimination reaction. (E1)
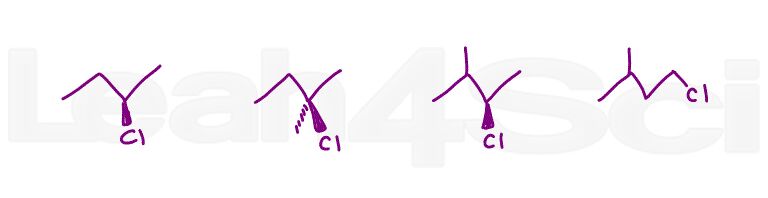
Question 3: Arrange the following molecules in order of increasing reactivity to undergo a bimolecular substitution reaction (SN2)
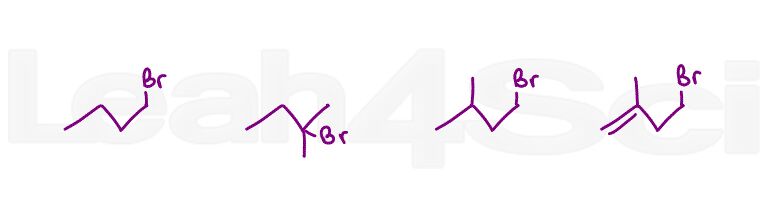
Question 4: Which direction is favored at equilibrium when dissolved in an acetone solution? What about methanol?
Need help? see the solvents video in the series

Predict the product for each of the following reactions. Be sure to show stereochemistry when appropriate. Be sure to label major/minor products when more than one outcome is possible.
Question 5:
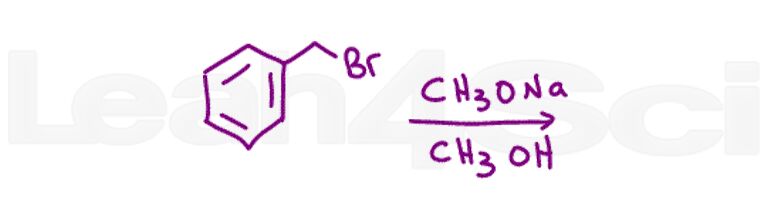
Question 6
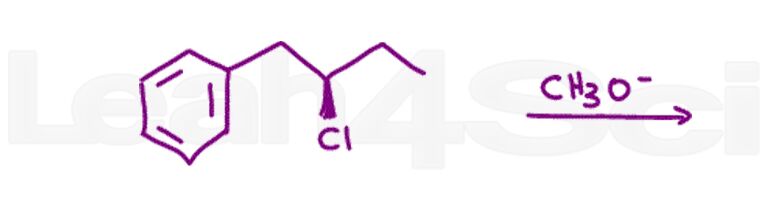
Question 7
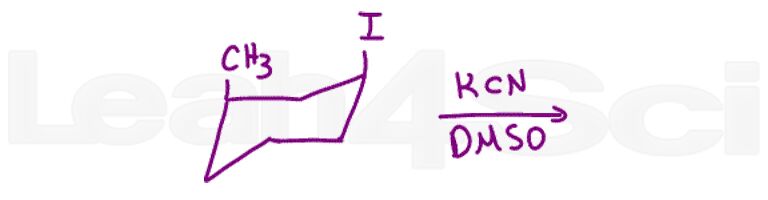
Question 8
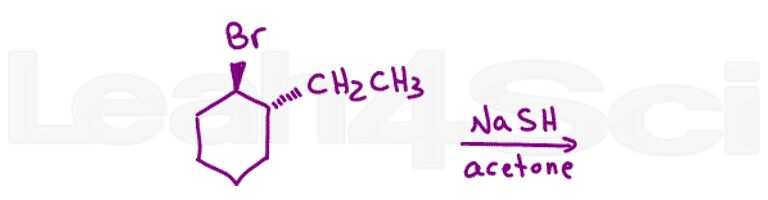
Question 9
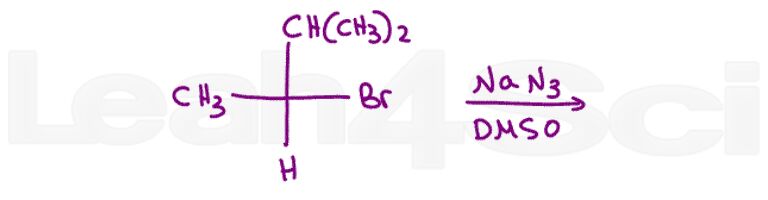
Question 10
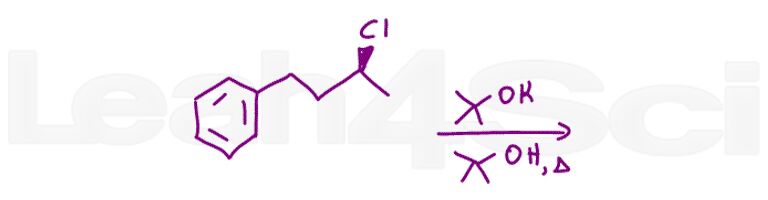
Question 11
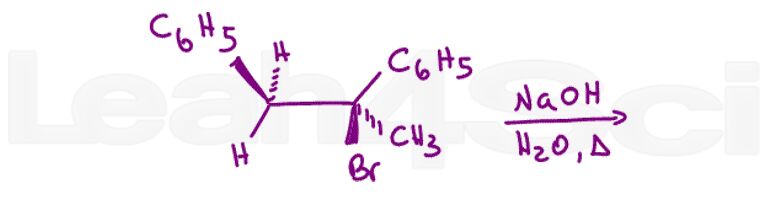
Question 12
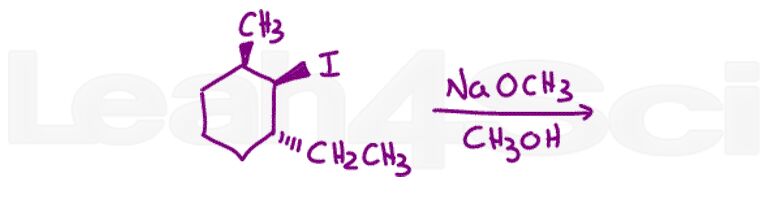
Question 13
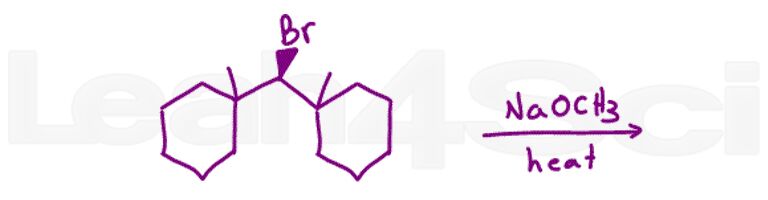
Question 14
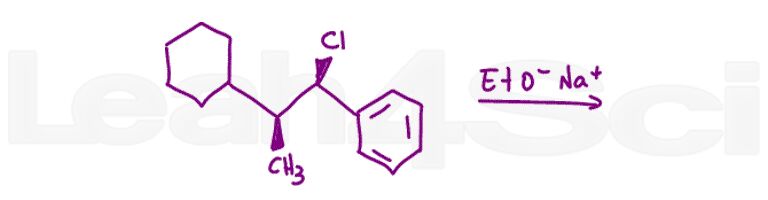
Question 15
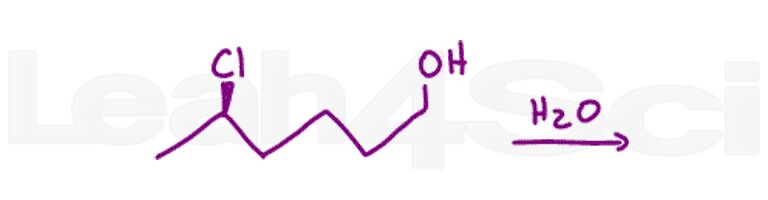
Question 16
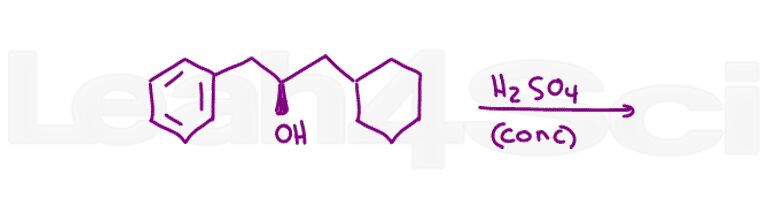
Question 17
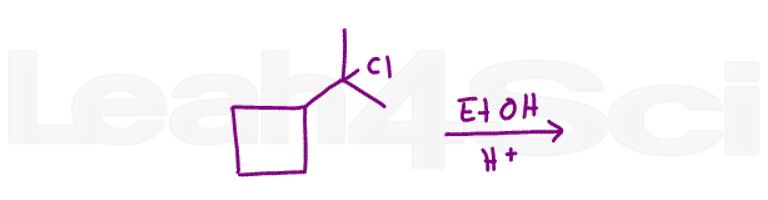
Question 18 Draw ALL possible starting haloalkanes that would form this product when undergoing unimolecular elimination.
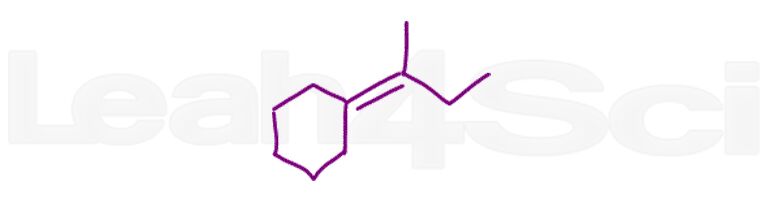
Question 19: Show how to bring about the following transformation. You may use any reactants and reagents necessary
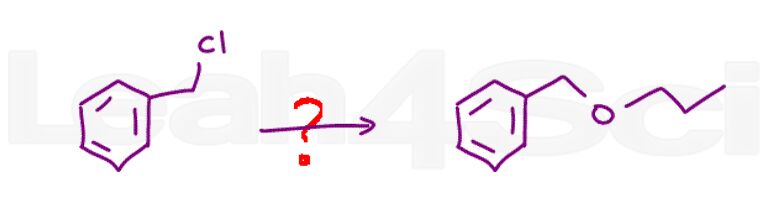
Question 20: Predict the product/s when (2R,3S) 3-ethyl-2-iodohexane reacts with NaSCH3 in dimethyl sulfoxide.
Question 21: Draw a complete mechanism for the following reaction. Be sure to include all intermediates, formal charges, and pushing arrows.
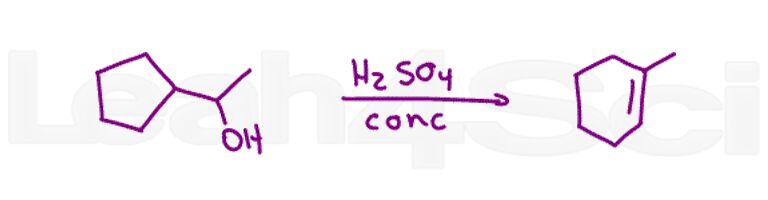
Bonus Question 22: Show how to bring about the following transformation. Hint: Pay attention to the orientation of substituents
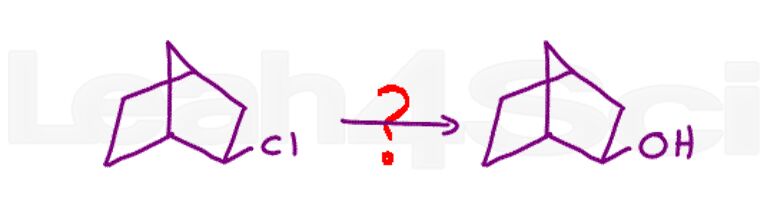
Bonus Question 23: Show the complete mechanism for the following transformation. Hint: More than one reaction is required.
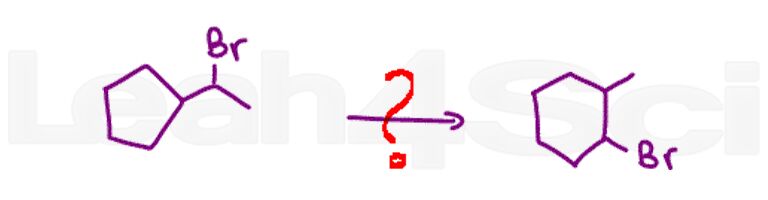
What did you think of this quiz? Easy? Hard? Need more practice?