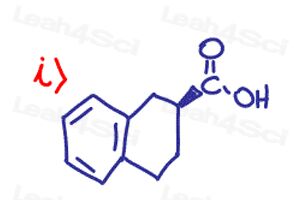
The key to mastering chirality and stereochemistry is through practice, practice, and more practice. But where do you get all those practice problems?
The key to mastering chirality and stereochemistry is through practice, practice, and more practice. But where do you get all those practice problems?
After reviewing the videos in my Chirality and Stereochemistry series, and working through the simple questions in each, see how well you’ve mastered the material by trying my Stereochemistry Practice Quiz below.
The questions range from medium to tricky to ENSURE you are comfortable looking out for those tricks. Be sure to review the videos first and follow along with the Stereochemistry Cheat Sheet.
(scroll down for the PDF solutions)
Stereochemistry Practice Part 1
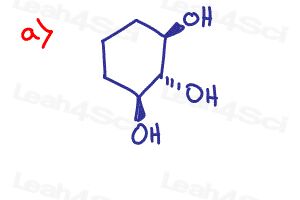
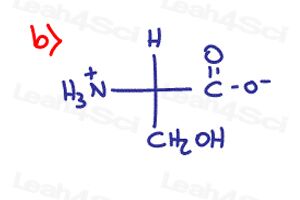
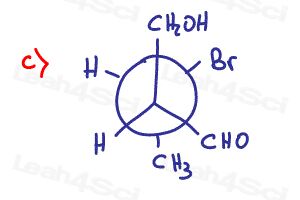
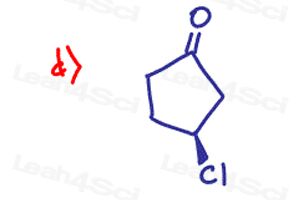
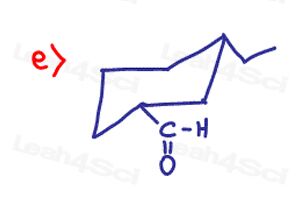
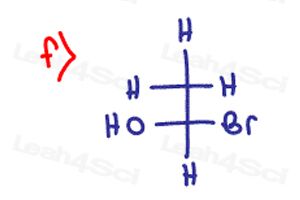
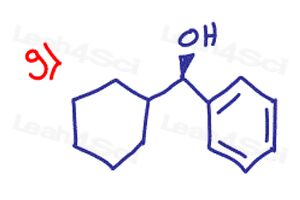
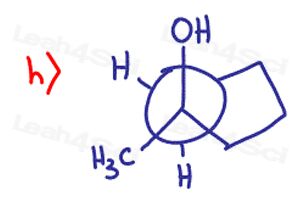
Determine if the following molecules are optically active (chiral) or optically inactive (achiral)
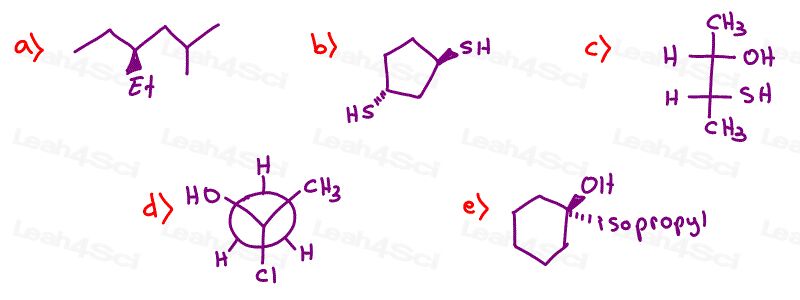
Stereochemistry Practice Part 2
Rank each set of substituents from high to low priority using Cahn-Ingold-Prelog ranking rules.
Hint: Need help ranking? See Chirality video 2
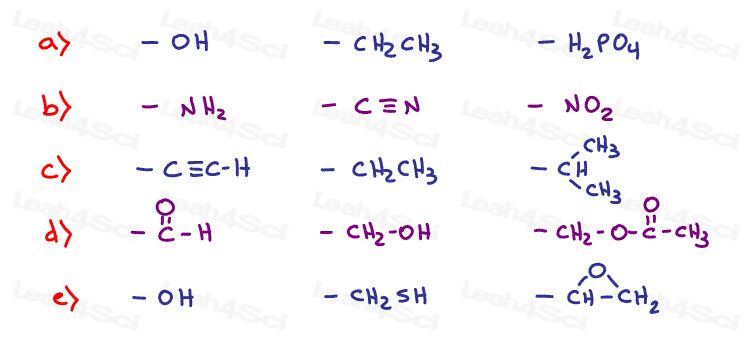
Stereochemistry Practice Part 3
Label every chiral carbon on the following molecules
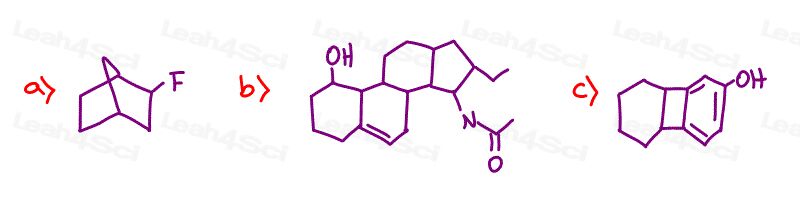
Stereochemistry Practice Part 4
Name the following compounds. Be sure to include Absolute Configuration (R/S) as applicable
Hint: Need a review on naming? See the Naming Organic Compounds video series
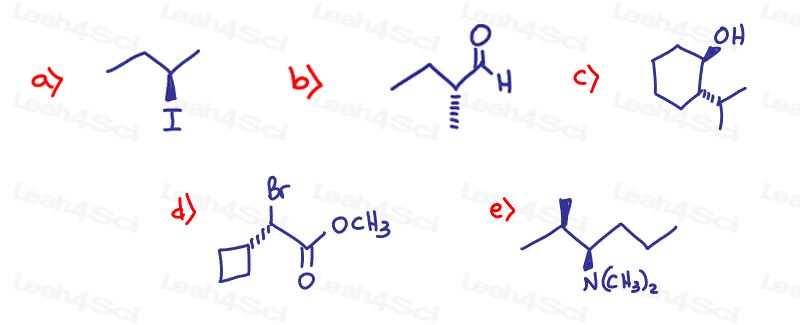
Stereochemistry Practice Part 5
Draw the enantiomer for each of the following chiral molecules
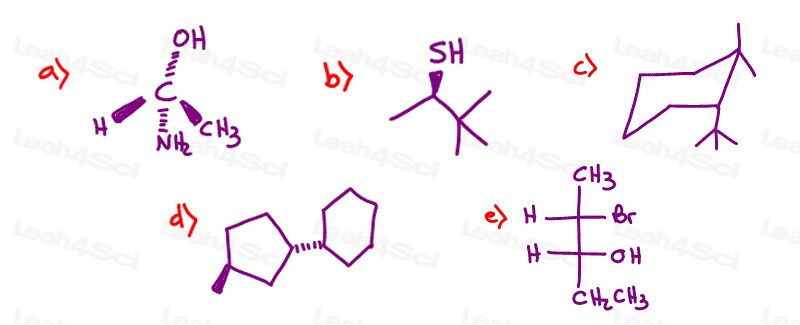
Stereochemistry Practice Part 6
Draw the following molecules clearly indicating every chiral center
- (S)-3-phenylpentanoic acid
- (R)-3,3-dimethyl-2-butanol
- (3R,4R)-3,4-dihydroxy-2-pentanone
Stereochemistry Practice Part 7
Find the absolute Configuration (R/S) for the following chiral compounds
Hint: Need a review on finding R and S? See videos 2-4 in the Chirality Series
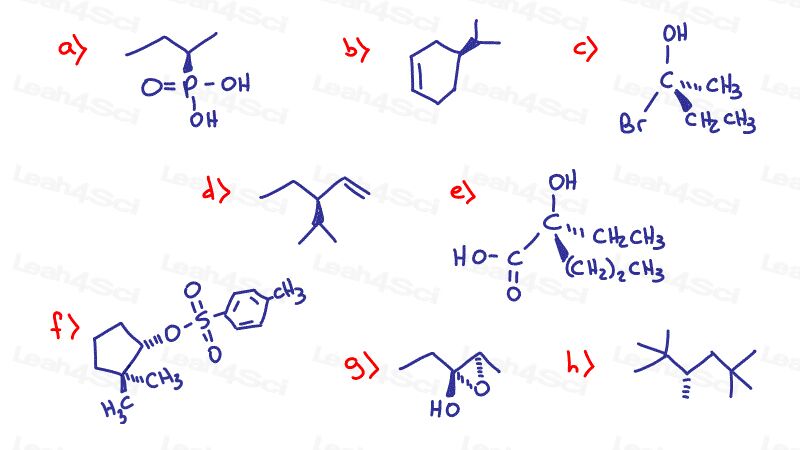
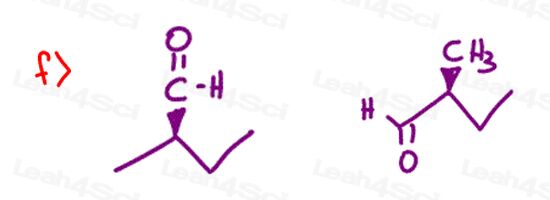
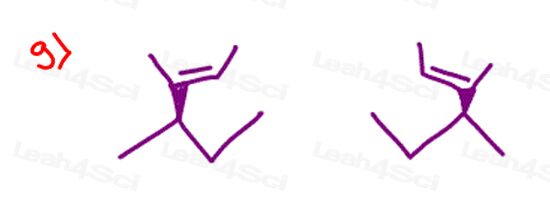
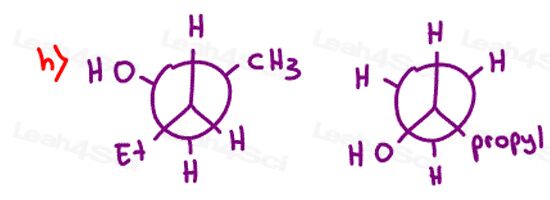
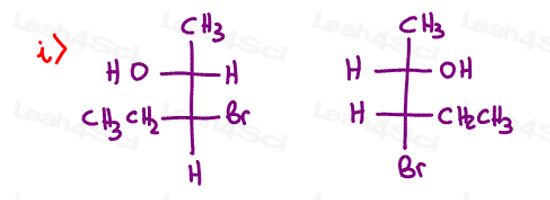
Stereochemistry Practice Part 8
Find the relationship between each molecule pair. Choose from:
- Identical / same compound
- Constitutional isomers
- Enantiomers
- Diastereomers
- Meso compounds
- Completely unrelated
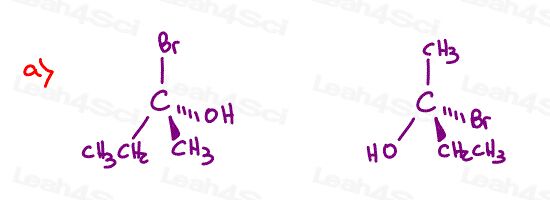
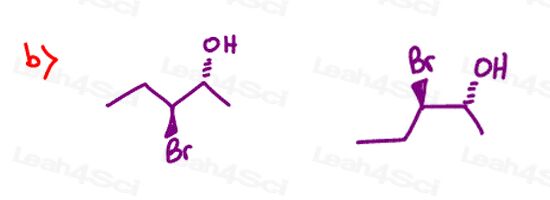
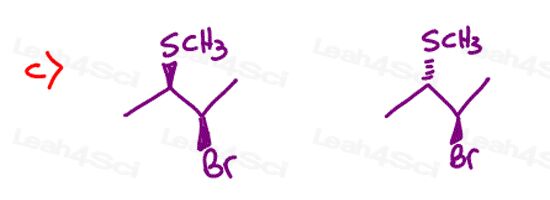
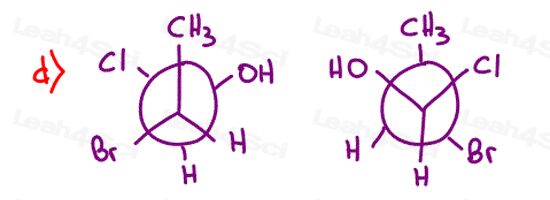
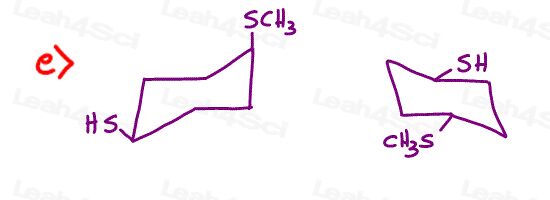
Stereochemistry Practice Part 9
Find the absolute configuration (R/S) for each chiral center on the following molecules. Hint: There may be more than 1 chiral carbon per molecule.
Stereochemistry Practice Part 10
Draw all possible stereoisomers for each of the following:
- 1,3-dibromocyclopentane
- 3,4,5-trimethyl-2-hexanone
- 3-isopropyl-4-methyl-2-pentanol
- 4-ethyl-3-methylhexanoic acid