 The key to mastering chirality and stereochemistry is through practice, practice, and more practice. But where do you get all those practice problems?
The key to mastering chirality and stereochemistry is through practice, practice, and more practice. But where do you get all those practice problems?
After reviewing the videos in my Chirality and Stereochemistry series, and working through the simple questions in each, see how well you’ve mastered the material by trying my Stereochemistry Practice Quiz below.
The questions range from medium to tricky to ENSURE you are comfortable looking out for those tricks. Be sure to review the videos first and follow along with the Stereochemistry Cheat Sheet.
(scroll down for the PDF solutions)
Stereochemistry Practice Part 1
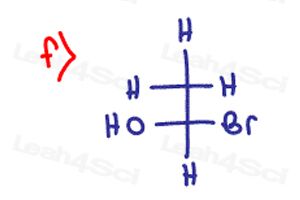
Determine if the following molecules are optically active (chiral) or optically inactive (achiral)
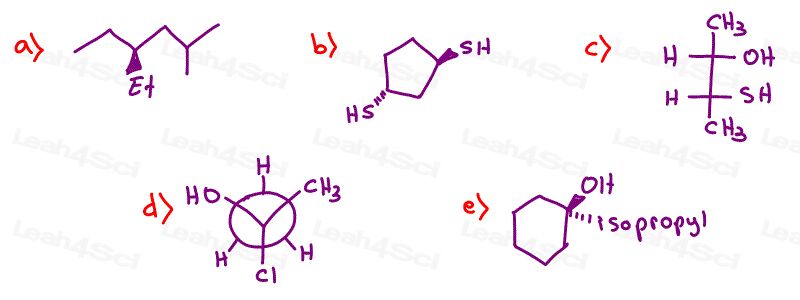
Stereochemistry Practice Part 2
Rank each set of substituents from high to low priority using Cahn-Ingold-Prelog ranking rules.
Hint: Need help ranking? See Chirality video 2
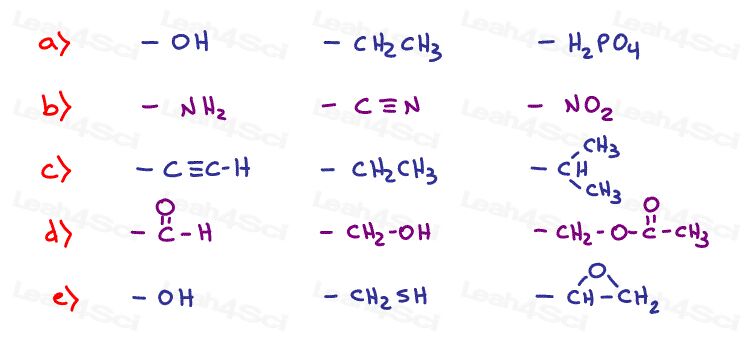
Stereochemistry Practice Part 3
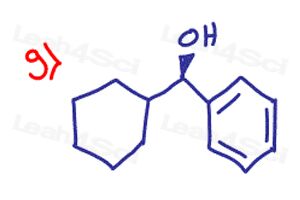
Label every chiral carbon on the following molecules
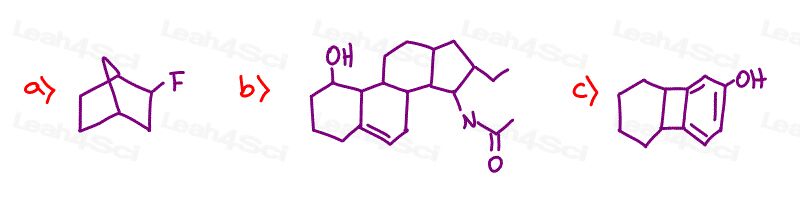
Stereochemistry Practice Part 4
Name the following compounds. Be sure to include Absolute Configuration (R/S) as applicable
Hint: Need a review on naming? See the Naming Organic Compounds video series
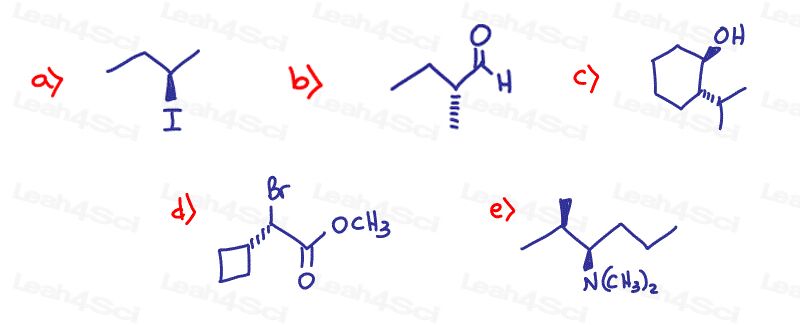
Stereochemistry Practice Part 5
Draw the enantiomer for each of the following chiral molecules
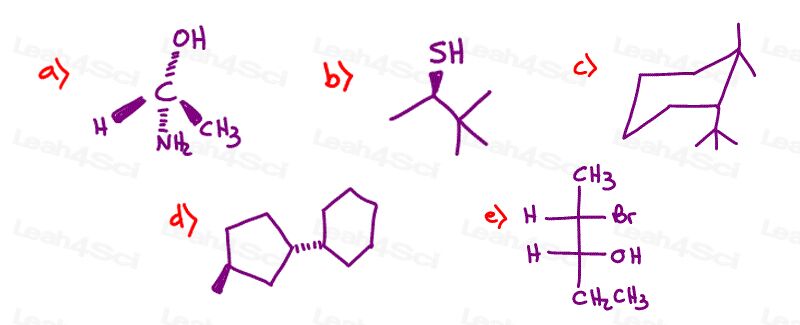
Stereochemistry Practice Part 6
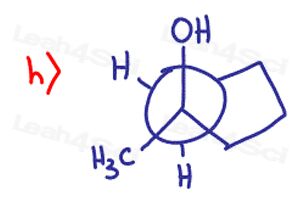
Draw the following molecules clearly indicating every chiral center
- (S)-3-phenylpentanoic acid
- (R)-3,3-dimethyl-2-butanol
- (3R,4R)-3,4-dihydroxy-2-pentanone
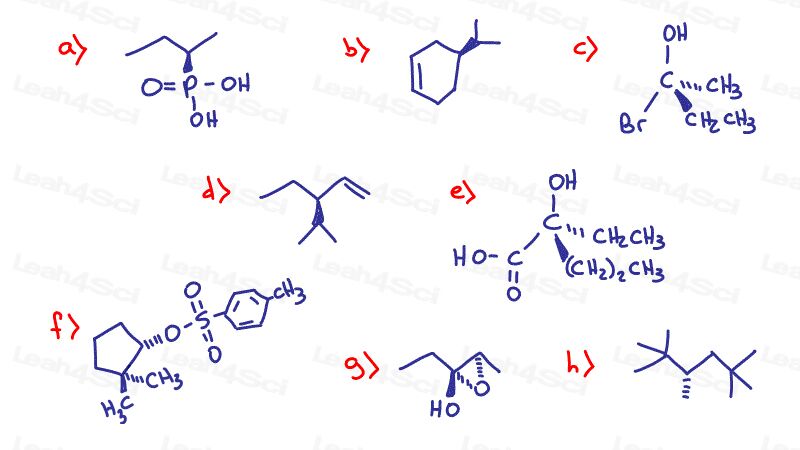
Stereochemistry Practice Part 7
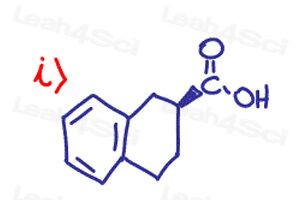
Find the absolute Configuration (R/S) for the following chiral compounds
Hint: Need a review on finding R and S? See videos 2-4 in the Chirality Series
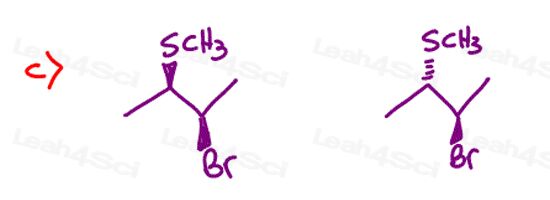
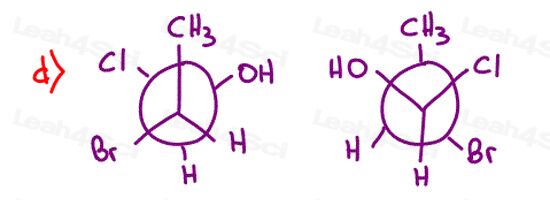
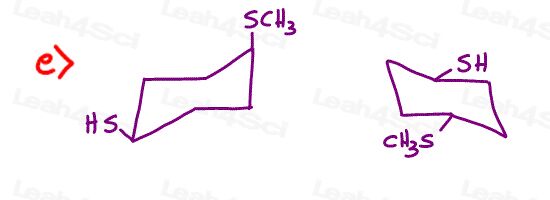
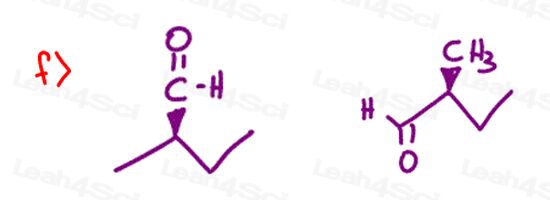
Stereochemistry Practice Part 8
Find the relationship between each molecule pair. Choose from:
- Identical / same compound
- Constitutional isomers
- Enantiomers
- Diastereomers
- Meso compounds
- Completely unrelated
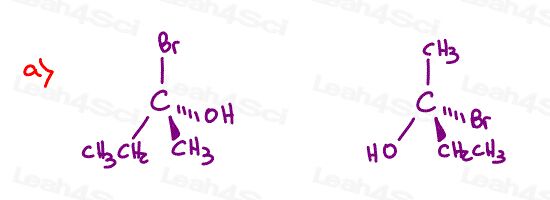
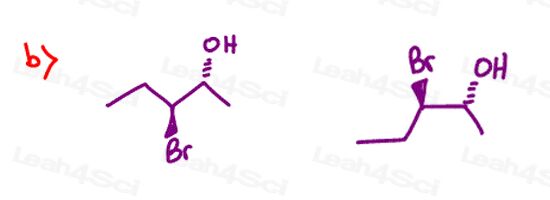
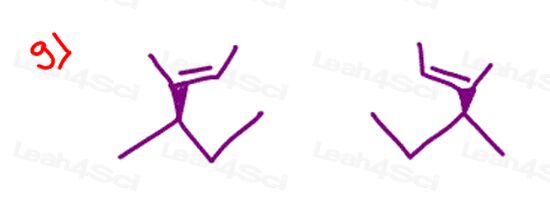
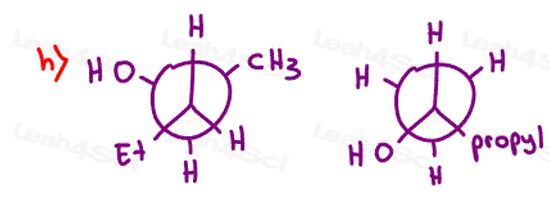
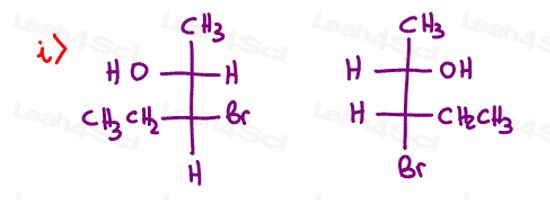
Stereochemistry Practice Part 9
Find the absolute configuration (R/S) for each chiral center on the following molecules. Hint: There may be more than 1 chiral carbon per molecule.
Stereochemistry Practice Part 10
Draw all possible stereoisomers for each of the following:
- 1,3-dibromocyclopentane
- 3,4,5-trimethyl-2-hexanone
- 3-isopropyl-4-methyl-2-pentanol
- 4-ethyl-3-methylhexanoic acid
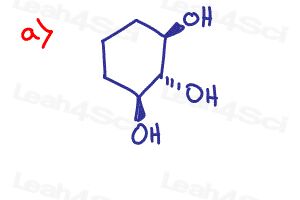


In solution paper answer of 10(part-3and 4) is missing.
Hello .am great .the site is so resourceful.thankyou
i did not get the solution can you help me?
sorry, i meant d part 8
how did h on part 8 end to be identical. i keep getting diastereomers for my answer
Can I know why part 1 b is a chiral molecule. Do we just take the mirror image?
were do i get the answers?
Great work dear.I will recommend this page to all my friends.
Thanks for all your work – it’s really great! 🙂
I’m confused about part 9 (d) – why is it R instead of S?
Thanks
Hi, Thank you so much for your amazing video, but where is the solution ? how I know if I am correct
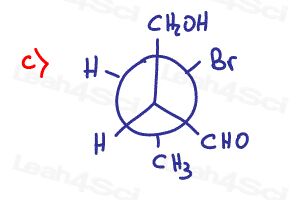
how is 8(b) becoming diastereomers?
very helpful,but i dont get it on how you chose the relationship between molecules,,any further help????
Why is (h.) in part 8 an enantiomer? There’s an ethyl group on one molecule and then a propyl group on the other. Can you explain please? Thank you so much!!
i love u mam:)) becoz of u i was able to score good markss……
Why does NH2 have a higher priority than CN?
Very helpful videos! Every explanation was very clear to follow, thanks a lot! Your swap method is awasome
Best teacher ever
Thank you so much for the illusioning questions. Enjoyed doing them with mistakes! Please add question banks for reagents, structural elucidation, organic spectroscopy and name reactions also. Please, please , please!!!!
For some reason I can’t seem to find the solutions even after giving my email.
hi
thank you so much! your a savior!
about qu’ 1:
why is a a-chiral??
Hi Leah. Thanks for the videos and material, they´re very helpful.
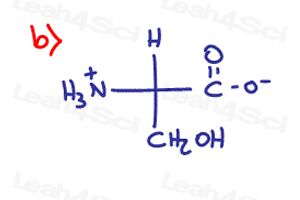
I have a question in part 9 b). When I order the groups Nh3 is the #1, COO- is #2 and CH2OH is#3, but with this order gives me an R configuration and in the solutions is S. I watched again the video for “R and S configuration for Lengthy Complex Substituents” but i still don´t understand.
Thanks again
In part three problem one, why are there 3 chiral carbons if none of them have unique substituents?
my bad, I meant only one of them is chiral because it has unique substituents. The two other carbons that are not attached to the F do not have unique subtituents
Hi , i still havent receive my pdf solution , how ?
I haven’t seen the solution PDFs since.
Please, kindly help with the solution.
How is part 1 a) Achiral? If I try to flip it, it puts the ET in the back, so it doesn’t superimpose. Could you help me understand please?
thank you so much Leah.I really found it helpful.Looking forward for new videos from you!
I already signed up and can’t get the solution… Why is everything and ad.
I didn’t get the chirality cheat sheet when I gave my email. It just sent me the whole cheat sheet pdf, which doesn’t include chirality. Help please?
I don’t know if you are still active on this but i’m trying to get the pdf solutions but it won’t show up in any of my emails. Could you try sending it again?
I am also having trouble with part 3, especially b and c. Could you please help me?
I do not understand why 1e is achiral.. please help
the solutions will never get emailed to you,dont waste ur time
Vry nice mam..really helpful..gud job
Hey, love your website and the way you explai!
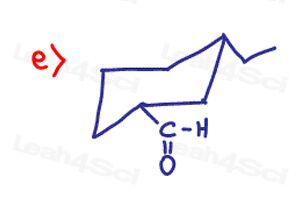
I have a question to part 8 of the quize, question e). I am confused, if it’s just cis and trans, i should just flip the ring to get to the right konformation right? But everytime i flip the ring axial goes to equatorial. At the first Ring there is a and e but in the secon ring, they are both equatorial. How can that happen?
Thank for your help.
Using your rules, I got the opposite answers for 9b,d,i. All of these examples, H is going into page right?
Also in 9h, what is the substituent group on the right that look like ]. Thanks in advance!
Can you please explain the R,S configuration in part 4(b)?
Could you help me with why 9b is s not r, i have the substituents ranked n^+h3 > coo^- > ch2oh > h and because my h facing backwards this would give an r configuration, thanks
In part 4 question e, why did you name the compound N,N? And also in ques b of same part why is it 1R,2S? Isn’t it 1R,1S?
Your videos are indeed a great help for those who want to master the topic.
Part 9 Part b…. why is it not R configuration? NH3+ gets the #1 priority, COO- gets the number 2, and CH2OH gets the number 3 priority. Since H is on a dash, we can just go straight clockwise from 1 -> 2 -> 3
Having a lot of trouble seeing how part 5 c) works x_x
How is the structure in the solution an enantiomer not a diastereomer?
I’m sorry, the solutions for part one dont align when the answers I got on chirality. I dont understand what Im doing wrong.
Which groups will be perpendicular to plan …horizontal or vertical???
How come the newman projection is achiral? the front carbon is attached to three different things isn’t it?
i love your work ….
want to meet you…
This was very helpful. Is there a video made purposely for this quiz? I would love to see where and how I messed up on some questions.
Check out my Chirality and Stereochemistry Tutorial Video Series: https://leah4sci.com/chirality-stereochemistry-enantiomers-diastereomers-r-s-organic-chemistry-tutorial-series/
practice was really helpful. Thanks a lot and really appreciate your work.
Zularnain, so glad you found this helpful!
Thanks for your resources they’re great! I’m confused about 1 thing… when do we use the swapping (ie. 2 swaps = same molecule) to determine R/S and when would we use just the switching to the opposite configuration when the lowest priority group is in the front??
One swap is the opposite. Two swaps gives you the same chirality.
If you do one swap, you have to remember to switch S to R or R to S. Sometimes I will forget that I only made one swap, therefore to avoid mistakes, I always do two so the chirality is the same.
why is (part 1 / a)) achiral? it has an H, ethyl, isobutyl and the Et (i don’t know what that means my the way :D), maybe it got something to do with that, because I am form Germany.
or is Et a short term for ethyl? that this makes sence
you forgot to draw the two methyl groups at part 7, f).. otherwise there would be no chiral center
7f only has one methyl group, or did you mean something else?
I too was worried about this at first. In your practice problem there are no methyl groups in the first cyclopentane whereas in your answer sheet there are two methyl groups. When i did the problem without looking at the answer sheet, there wasn’t a chiral molecule. But in the answer sheet the C=O is S BECAUSE there are two methyl groups in the cyclopentane that wasn’t present in the practice problem.
The ethyl group thats visible and the Et that is branched are actually both ethyl groups. That means this molecule only has three unique substituents making it achiral. I purposefully drew it this way so that you are not fooled when your professors try to pretend you have four unique groups but there are actually only three, like in this question.
Thanks for all this tutorials.it helped me lot .
You’re welcome Arijit
I like it. But no pdf notes?
Did you sign up for it Israel?
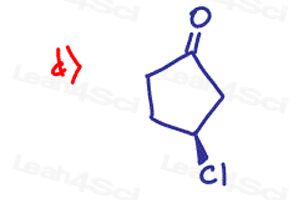
For Stereochemistry Practice Part 9 letter D:
why is it not the S configuration? Cl gets the highest priority, afterwards the side closest to the double bond oxygen gets the next priority and then the side furthest from the double bond oxygen gets the 3rd priority and since H is in the back it makes a counterclockwise arrow and so should it not be S?
I was running into the same problem with B as well.
I’m not sure what I’m doing wrong, since my answers for B and D did not match the answer key.
Also thank you for the amazing tutorials, I wish I found your site earlier!
Thank you so much! I am taking Orgo I right now and it helps me a lot. 🙂
Thanks Pamela, glad to help
*sad* can’t get the PDF solution coz I live in Africa. Pleasssssee Leah is it possible to send it to my email. Your site is the bomb!!! telling all my friends about it.
You should be able to get the PDF anywhere in the world unless your computer is blocking the email Jaey
I love this…how I wished I had known this site before..ahhh
Better late than never, right Busari? Share with your classmates so they don’t have similar regrets
THIS IS DIFFICULT !
That’s the point 😉
you promised to send the pdf solution and its been a while since. Kindly please assist. thank you.
It should have arrived in your email inbox with 5 minutes. Check your spam box and junk folders
these are awesome problems!! they have so much diversity and cover all aspects of all possible “tricky”questions. Please put up the solutions
haha thanks Denice. The solutions are linked at the end of the quiz
thanks
your help it means a lot to me
You’re very welcome Amina