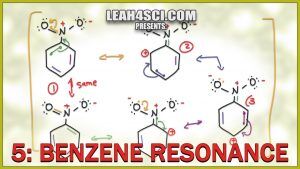
 Benzene is a unique molecule when it comes to resonance structures.
Benzene is a unique molecule when it comes to resonance structures.
This video will show you how to draw the ‘circle' of resonance for benzene, as well as resonance intermediates for substituted aromatic compounds including Electron Donating Groups EDG which resonate into the ring and Electron Withdrawing Groups EWG which cause resonance out of the ring.
Turn benzene into easy points on your next exam by learning how to double check yourself with the formal charge shortcuts and proper arrow drawing.
(Watch on YouTube: Benzene. Click cc on bottom right for video transcription.)
<– Watch Previous Video: Drawing Radical Resonance for Allylic and Benzylic Radicals
–> Watch Next Video: Resonance Structures Practice Solutions
Links & Resources Mentioned In This Video:
- Formal Charge Formula Short Cut Written Tutorial
- Formal Charge Formula Short Cut Video Tutorial
- Resonance Practice Quiz



Thanks a lot to you dear , Iam trying to pass the exams and understand organic chemistry by your videos , iam study at college of science/chemistry department
For the benzene ring with NO2, is it possible for there to be more resonance structures besides the ones shown above? I was able to draw other resonance structures, but then I recalled from one of the previous videos that some of the resonance structures may not be significant.