 Resonance structures are required throughout organic chemistry. You'll learn how to draw resonance early in orgo 1, and be tested on resonance intermediates in advanced orgo 2 mechanisms.
Resonance structures are required throughout organic chemistry. You'll learn how to draw resonance early in orgo 1, and be tested on resonance intermediates in advanced orgo 2 mechanisms.
To help you take away the guesswork I've put together a brand new series taking you through the basics, starting with the question: What IS Resonance, all the way to rules, tips, tricks, practice, and even radical resonance.
Think you've mastered it all? Jump right to my Resonance Structures Practice Quiz
Included in this series:
- What is Resonance
- Formal Charge Formula Shortcut
- Key Arrow Patterns in Resonance Structures
- Major and Minor Resonance Structures
- Radical Resonance
- Resonance Structures of Benzene
- Resonance Structures Practice Solutions
Video 1 – What is Resonance?
 The first step to mastery is understanding. Don't just memorize ‘these arrows move here'. Instead, make sure you get why molecules resonate to delocalize their electrons, and understand the difference between ‘resonance hybrid' and ‘resonance intermediates'. This and more is covered in Video 1 of this mini series.
The first step to mastery is understanding. Don't just memorize ‘these arrows move here'. Instead, make sure you get why molecules resonate to delocalize their electrons, and understand the difference between ‘resonance hybrid' and ‘resonance intermediates'. This and more is covered in Video 1 of this mini series.
Formal Charge in Resonance
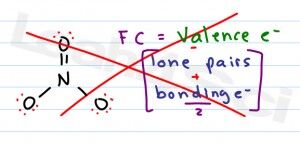 When drawing resonance structures you'll have to keep an eye on the change in formal charge. But you won't have time to go through the lengthy/complex equation in your book. Instead be sure to utilize my Formal Charge Formula Shortcut.
When drawing resonance structures you'll have to keep an eye on the change in formal charge. But you won't have time to go through the lengthy/complex equation in your book. Instead be sure to utilize my Formal Charge Formula Shortcut.
Video 2 – Key Arrow Patterns in Resonance Structures
 Now that you have a resonance foundation, it's time to put your curved arrows to work.
Now that you have a resonance foundation, it's time to put your curved arrows to work.
They key to finding resonance structures, is knowing which electrons to move, WHERE to move them, and WHAT NOT TO DO!
Video 3 – Major and Minor Resonance Structures
Not all resonance structures are created equally.
While an arrow CAN move onto a given atom, doesn't mean it WANTS to. Once you've come up with all possible resonance forms, you'll have to understand the stability of each structure to determine which are major and which are minor.
Video 4 – Radical Resonance
 When you think of resonance, you're likely thinking of 2 electrons as a lone pair or pi bond. But radicals, or unpaired single electrons can also participate in resonance to increase their stability.
When you think of resonance, you're likely thinking of 2 electrons as a lone pair or pi bond. But radicals, or unpaired single electrons can also participate in resonance to increase their stability.
Video 4 shows you how to draw the ‘fishhook' resonance for allylic and benzylic radical structures
Video 5 – Resonance Structures of Benzene
 Benzene is a unique molecule when it comes to resonance structures.
Benzene is a unique molecule when it comes to resonance structures.
Learn how to draw its resonance, as well as resonance intermediates for substituted aromatic compounds including Electron Donating Groups which resonate into the ring and Electron Withdrawing Groups which cause resonance out of the ring.
Video 6 – Resonance Structures Practice Solutions
First, complete the Resonance Structures Practice Quiz, and then watch this video where I go over the first three question solutions and explanations step-by-step!
Having gone through this series, how do you feel about resonance structures? If you still have questions, WATCH THE SERIES AGAIN! If you feel confident then test your understanding by trying my free Resonance Structures Practice Quiz.



