 This Tutorial Video Series will guide you through the content you need to master for MCAT Chemistry Stoichiometry and Reactions. This series will help you master AAMC Content Category 4E!
This Tutorial Video Series will guide you through the content you need to master for MCAT Chemistry Stoichiometry and Reactions. This series will help you master AAMC Content Category 4E!
This series includes everything from basic overviews of molecules and calculations, to advanced reactions, balancing and more.
You'll also learn the most important aspect, the math and shortcuts behind this topic. Still rusty? Start with the MCAT Math Without A Calculator and MCAT Conversions tutorial series.
It will cover everything from terminology, step by step explanations, and practice problems!
Don't forget to grab your free copy of my Strong Acid/Base Mini Cheat Sheet here to help with your memorization.
Test your Mastery with my NEW Stoichiometry & Reactions Practice Quiz!
Video 1: Intro to Reactions & Stoichiometry
 Start with a quick overview of key terms and concepts to build a proper foundation for the remainder of this series.
Start with a quick overview of key terms and concepts to build a proper foundation for the remainder of this series.
If you don't understand the terms, you'll have trouble with the related math. Can't skip this one!
Video 2: Calculating Molecular Weight and Formula Weight
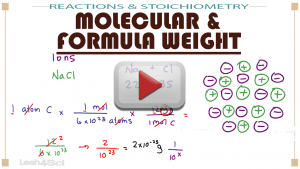 This video will show you how to find molecular weight in AMU, grams per mole, formula weight conversions, and more.
This video will show you how to find molecular weight in AMU, grams per mole, formula weight conversions, and more.
Best of all, you'll learn how to do this QUICKLY,
Despite not being allowed to use a Calculator on the MCAT.
Video 3: Empirical Formula and Molecular Formula Calculations
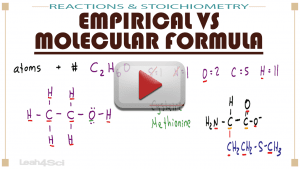 This video explains the definitions, similarities, and differences between an atom's empirical formula and its molecular formula.
This video explains the definitions, similarities, and differences between an atom's empirical formula and its molecular formula.
You'll also learn how to calculate each with simple and easy-to-apply logic.
Video 4: Molarity, Molality, and Molar Mass
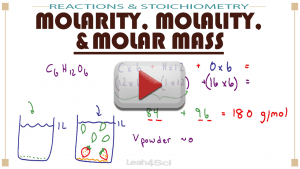 Learn the definitions, calculations, and how the difference between molarity and molality.
Learn the definitions, calculations, and how the difference between molarity and molality.
Also learn a time saving shortcut to quickly interconvert between molarity and molality on the MCAT.
Video 5: Percent By Mass Composition
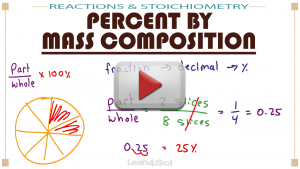 Learn ONE rule for all these types of questions!
Learn ONE rule for all these types of questions!
This video makes this concept simple. You’ll see WHY and HOW these equations work, instead of memorizing all the different percent formulas.
Video 6: Mole and Avogadro's Number
 This video covers the definition of Moles as well as an illustration showing the logic of moles in general chemistry.
This video covers the definition of Moles as well as an illustration showing the logic of moles in general chemistry.
You’ll also see how to easily and quickly convert from and to them and other units on the MCAT!
Video 7: Density
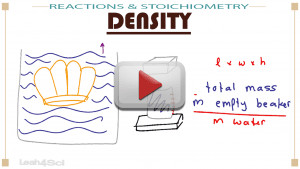 This video covers the definition in Chemistry vs in Physics, the logic behind the equations, and step-by-step solutions to practice problems!
This video covers the definition in Chemistry vs in Physics, the logic behind the equations, and step-by-step solutions to practice problems!
Video 8: Oxidation Numbers
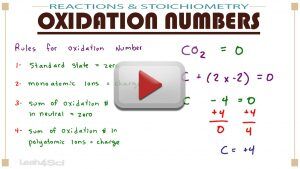
Learn what they are, how to find them on simple molecules/ions, and how to calculate oxidation numbers for atoms in complex molecules and polyatomic ions.
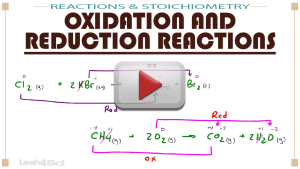
Video 9: Oxidation and Reduction Reactions
Oxidation and Reduction Reactions: Learn what they are, how to identify oxidation and reduction half reactions, and how to recognize what gets oxidized or reduced in complex reactions including a time-saving shortcut.
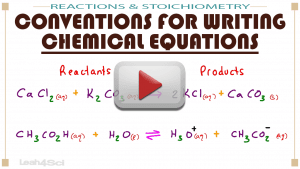
Video 10: Conventions for Writing Chemical Equations
When it comes to chemical reactions it’s very easy to confuse all of the numbers and letters: Which go up or down? Which go in the parenthesis or out? This video breaks it all down from arrows and phases to coefficients, numbers for ions and polyatomic ions.
Video 11: Common Types of Chemical Reactions
Common types of chemical reactions that will show up on the MCAT include: Disproportionation, Single Replacement, Double Replacement, Combination or Synthesis, Decomposition, and Combustion
Video 12: Balancing Chemical Equations
 Stoichiometry or balancing of chemical equations can be tedious and confusing unless you have an efficient system to follow. These (3) videos will teach you a simple approach and a few time-saving shortcuts for balancing reactions from simple to more complex such as acid/base, ionic, and combustion reactions
Stoichiometry or balancing of chemical equations can be tedious and confusing unless you have an efficient system to follow. These (3) videos will teach you a simple approach and a few time-saving shortcuts for balancing reactions from simple to more complex such as acid/base, ionic, and combustion reactions
Video 13: Balancing Redox Reactions
Balancing Redox Reactions requires balancing of atoms AND electrons. This video will teach you how to balance atoms and charge/electrons in oxidation and reduction reactions, then how balance redox reactions in both acidic and basic conditions.
Video 14: Limiting Reactant, Excess Reagent and Product Yield
In this video, I’ll show you how to understand the concept behind limiting reactants from a logical perspective by working a simple, real-world example. I’ll offer two different approaches to determining the limiting reactant using stoichiometric ratios. Additionally, you’ll learn how to calculate product yield and the amount of excess reagent.
 How do you feel? Test your understanding with the new MCAT Reactions and Stoichiometry practice quiz!
How do you feel? Test your understanding with the new MCAT Reactions and Stoichiometry practice quiz!
Want more MCAT Tutorials? See the MCAT Homepage