 Electrochemistry, as the name implies, is a topic that deals with the relationship between electrical potential and chemical reactions.
Electrochemistry, as the name implies, is a topic that deals with the relationship between electrical potential and chemical reactions.
Why is that relationship important, you ask? We can use electrical current to drive a nonspontaneous chemical reaction, or we can use a spontaneous reaction to drive an electric current (and store it!).
And since electrochemistry is required for the MCAT, you need to be familiar with electrochemical cells – what they are, how they work and even some of the math that goes along with them.
What confused me most in undergrad, and also confused many of my current MCAT students, was distinguishing between the galvanic/voltaic cell and the electrolytic cell. Especially when they showed up during bio lab experiments (Electrophoresis after PCR).
Because yes, you WILL be expected to know the differences between the two, describe how each works, and draw out their components.
Electrochemistry Foundations
Before we jump into electrochemical cells, let’s revisit a few key foundational topics:
- Redox reactions to understand what happens in the cell
- Assigning oxidation numbers (Trust me, you’ll need it for the later math.)
- And of course, balancing redox reactions.
Now that we have the basics, let’s jump in.
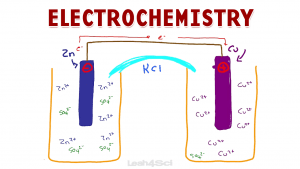
Video 1: Galvanic/Voltaic Cells
Let’s start with an overview of the electrochemical cell driven by a spontaneous redox reaction.
The video below walks you through everything from the overall Galvanic (also called Voltaic) cell, the individual half-cells and salt bridge.
You’ll learn about the flow of electrons between the anode and cathode as well as how to figure out where and how oxidation and reduction take place.
Video 2: Electrolytic Cells
The Electrolytic cell (unlike what we discussed above) is NOT driven by a spontaneous reaction and therefore requires an external electrical current.
Watch below to learn about this nonspontaneous electrolytic cell, how it works, what drives the reverse chemical reaction, and how it differs from the galvanic cell above
Video 3: Galvanic/Voltaic vs. Electrolytic Cells
This video gives you an in-depth comparison of the galvanic/voltaic electrochemical cell and the electrolytic cell. You'll review the components of both cells and see exactly what’s happening with each species in the reaction, where the electrons are flowing and how the cell is being powered. Most importantly, in watching this video, you'll learn to point out the similarities and differences between the two cells, including the charges on the electrodes.



Leave a Reply