 Substitution and Elimination reactions are potentially the most difficult topic at the Organic Chemistry 1 Level. Unlike other reactions which follow similar patterns, with the SN1/SN2/E1/E2 reactions you are faced with different circumstances for similar molecules and asked to choose a reaction pathway.
Substitution and Elimination reactions are potentially the most difficult topic at the Organic Chemistry 1 Level. Unlike other reactions which follow similar patterns, with the SN1/SN2/E1/E2 reactions you are faced with different circumstances for similar molecules and asked to choose a reaction pathway.
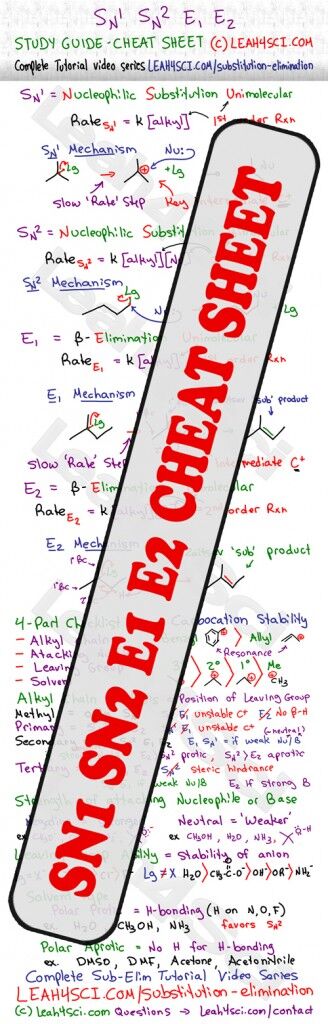
The average cheat sheet gives you a ‘memorize without logic' roadmap, which in my opinion, does NOT help for tricky questions. And so I've put together this ‘logic-based' cheat sheet, to help you understand and recognize what you're looking at with reactions. This is a quick reference, for detailed tutorials on each individual concept, 20+ video series on Substitution and Elimination reactions
I put in countless hours to compile this cheat sheet. If you find it helpful please share using the facebook/twitter… icons above, and leave a comment below letting me know what you like most about it.
Click for full version



Thank you so much for your help Leah!
Great!
Thank you so much. It make me easy to learn.
This was really helpful…thx fr talking such a great initiative! 🙂
dear leah, i cant download the cheat sheets, why ?
Thanks a lot, I have my 1st semester exam after two days, and I dont know much. Its really helpful to me.
I can’t believe such a beautiful paper existed. THANK YOU TO THE MOON AND BAAAAAACK <3 <3
Awesome cheat sheet for the new comers…..IT will be great for MCQ type questions…But for subjective plz go through the concepts
in E2 reactions, t if it react with Bulky base (t-BuOK, diisopropylamine, or triethylamine) –> products should be Hoffman (major) and Zaisev (minor), but with (EtO- ) –> products will be Hoffman (minor) and Zaisev (major)
I just saw on your video only discuss about Zaitsev product, but it shoule be 2 products each reaction?
Leah, would you be able to make these into a print-friendly version for future students? I’ve printed this one (also the Alkene reactions) out and some of the information was cut off since the image is so large.
Bren if you download the PDF it’s formatted to fit a standard print page
Agree with commenter above. You state polar protic solvents favor SN2. This is incorrect. A protic solvent will form hydrogen bonds and solvate a nucleophile, thus slowing down the SN2 by forcing the breaking of hydrogen bonds. Polar *a*protic solvents are used to encourage SN2.
You are correct, the error came when moving parts around on the cheat sheet. Sorry for the misunderstandings. I will correct it shortly
Great summary. Really useful for a quick overview.
Thank you Rukshi
I believe that there is a typo in this cheat sheet, Polar Aprotic Solvents, like acetone and DMSO, will favor Sn2 as opposed to Sn1. It is correct the first time, but towards the bottom it appears mislabeled.
You’re right Christian. I think I tried to squish too much into the tiny space. Hopefully you got a ton of value from the rest of the cheat sheet