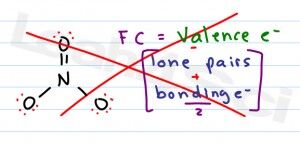
 Formal Charge is that pesky concept covered early in organic chemistry and somehow never seems to go away.
Formal Charge is that pesky concept covered early in organic chemistry and somehow never seems to go away.
Why do I call it pesky?
Because the equation in your textbook is long, confusing, and needlessly annoying.
How can you afford that kind of time when working through a multi-step synthesis?
You Can’t!
You shouldn’t have to.
Yet understanding the nature of Formal Charge is a critical component when it comes to mastering organic chemistry reactions and mechanisms. Formal charge helps you understand reaction patterns by showing you why a specific atom attacks, and why its ‘victim’ accepts the attack.
So what exactly IS formal charge?
Formal charge is the actual charge on an individual atom within a larger molecule or polyatomic ion.
The sum of formal charges on any molecule or ion results in the net overall charge.
This concept is simple enough for small ions. Chloride obviously has a negative charge. Even the negative charge on the hydroxide oxygen is simple to understand.
But what if you have a much larger group of bound atoms with an overall net charge? For example, the negative nitrate or triple negative phosphate.
Where does the charge come from?
Which specific atom is responsible for the overall charge?
Can it be more than one atom?
And, what if you’re studying an uncharged molecule – Is it still possible for individual atoms to carry charge despite the net neutrality?
The answers are yes, yes, yes, and more yes.
Think back to general chemistry when you studied ion formation. An ion is simply an atom or molecule that gained or lost electrons to get a net charge.
If the atom has just one more negative electron, than protons, it will have a net negative charge.
If the atom loses negative electrons and therefore has as few as one extra POSITIVE protons in its nucleus, it will carry a net positive charge.
Now that you know WHAT we’re studying, let’s see HOW! – That’s where I can help you.
How to Calculate Formal Charge
When I first studied formal charge I was lost. The formula in my textbook was long, tedious and brutal. A long, annoying trip to my professor’s office hours enabled me to figure it out, but it was still overwhelmingly TEDIOUS!
Let’s start with the proper formula and then learn a shortcut.
Formal Charge Equation
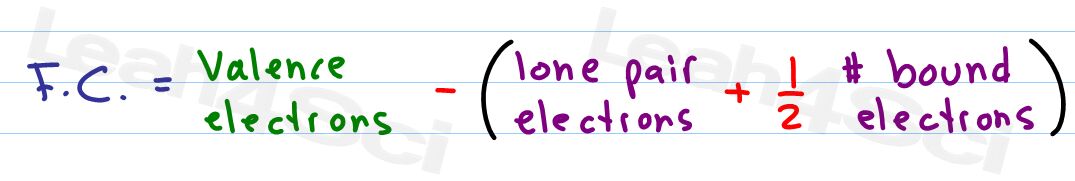
Formal Charge = [# valence electrons on neutral atom] – [(# lone electron pairs) + (½ # bonding electrons)]
- Valence electrons = corresponds to the group number of the periodic table (for representative elements).
- Lone Pairs = lone electrons sitting on the atom. Each electron counts as one and so a pair counts as two.
- ½ # bonding electrons = Since a bond is formed by sharing 2 electrons between 2 atoms, every atom in the bond can only take credit for one of the 2 electrons. A double bond containing four electrons allows each atom to claim 2. A triple bond of 6 electrons allows each atom to claim three.
Are you confused?
Do you dread running through this every time you finish a reaction?
I’m with you.
Yes the equation works, but it’s far too tedious and annoying! There are just too many steps and calculations.
Here another option, MY version – one that is easier, faster, and comes out with the same result!
Formal Charge Calculation Shortcut
Here’s my version of this equation.
Ready?
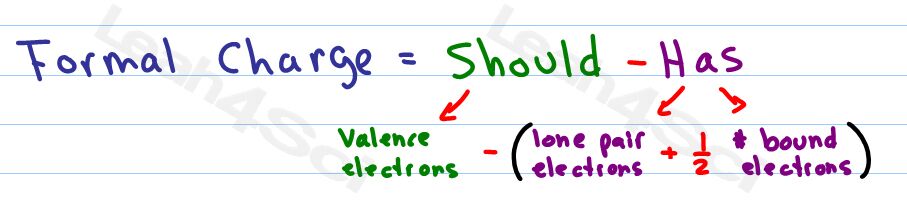
Formal Charge = Should – Has
Definitely faster, right?
This shortcut is guaranteed to save precious seconds on your exam IF AND ONLY IF you understand how to apply it.
But when you understand it you’ll be able to solve formal charge in your head, in under 8 seconds per atom.
Let’s make sure you understand this shortcut
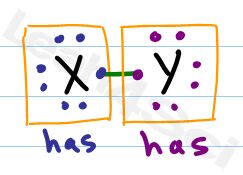
Should = the number of valence electrons that a neutral atom SHOULD have.
Has = the number of electrons an atom HAS directly attached, touching the atom in question.
Lone pairs represent 2 electrons sitting on the atom so that Has = 2
Each bond only counts for a single electron since the second electron in the bond is touching the other atom.
Want to see this shortcut brought to life? See my formal charge video below
When you first study formal charge it helps to draw out the Lewis Structure for every molecule in question. As you get more comfortable with this topic you’ll be able to pick out bonds on skeletal bond-line drawings.
For now, let’s stick with the basics (or better – Let’s keep it simple), and look at a handful of examples utilizing the checklist shared in the Lewis Structures video below. This is a must-learn checklist – so if you didn’t watch yet, review this video:
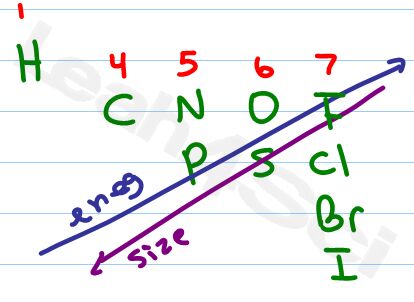 As you work through Lewis Structures it helps to have a periodic table handy. Since we are working on saving you time though, your most efficient results will involve you memorizing the following 10 atoms. As you memorize their order and location, pay particular attention to these trends:
As you work through Lewis Structures it helps to have a periodic table handy. Since we are working on saving you time though, your most efficient results will involve you memorizing the following 10 atoms. As you memorize their order and location, pay particular attention to these trends:
- Group numbers as written on top
- Electronegativity increases up and to the right
- Size increases going down and to the left
Let’s start with an easier example. We’ve already discussed hydroxide so let’s take a look at the OH- Lewis Structure.
Following the checklist in the Lewis Structures video we have oxygen bound to hydrogen, with 3 lone pairs around oxygen.
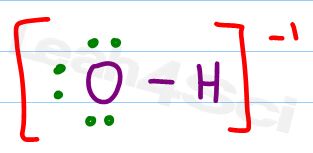
Let’s apply the shortcut:
Hydrogen should have 1 valence electron
Hydrogen has one attached electron
1 – 1 = 0
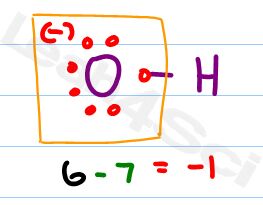 Here’s a trick for bound hydrogen, it ALWAYS has a formal charge of zero, so just ignore it.
Here’s a trick for bound hydrogen, it ALWAYS has a formal charge of zero, so just ignore it.
Neutral oxygen should have 6 valence electrons
Oxygen has 7 attached electrons as shown in red
Should – Has = 6 – 7 = -1
Hydroxide has a negative formal charge on the oxygen atom.
What about Nitrate?
Let’s analyze the NO3- Lewis Structure and Formal Charge
Following the checklist we draw our atoms, bonds, and electrons.
Due to resonance we would show three structures for nitrate. However we’ll just look at one for the sake of our experiment.
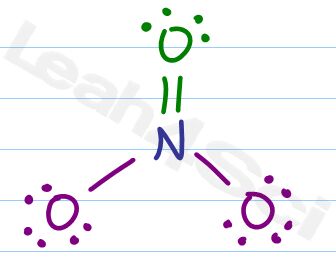
Now for formal charge
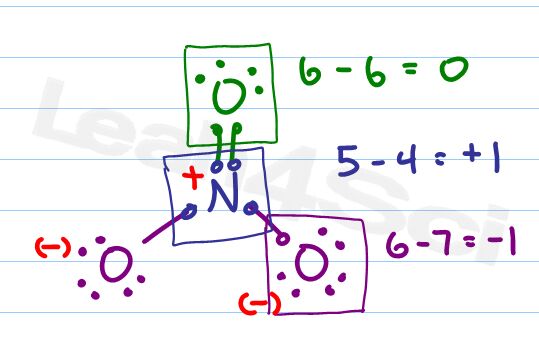 Should – Has = 5 – 4 = +1
Should – Has = 5 – 4 = +1
Neutral nitrogen should have 5 valence electrons but our drawing only shows 4 attached.
Next we’ll look at the double bound oxygen.
Should – has = 6 – 6 = 0
The double bound oxygen is happy, stable, and has a net neutral charge.
Finally we have 2 single bound oxygen atoms with 3 lone pairs each. Since they are identical we have to calculate just one to get the answer for both.
Should – Has = 6 – 7 = -1
Oxygen should have 6 valence electrons, each of the remaining oxygen atoms have 7 attached electrons for a net negative charge.
Let’s try one more simple yet tricky example. Carbon monoxide.
Simple because it only has 2 atoms.
Tricky because carbon monoxide is neutral, but don’t let this fool you. Many a hemoglobin has been fooled by CO making it such a deadly poison.
There are 2 types of molecules with a net zero charge
- molecules with no formal charge
- molecules with formal charge that cancel out for a net zero formal charge.
CO Lewis Structure and Formal Charge
 Start with the Lewis Structure checklist
Start with the Lewis Structure checklist
Now let’s tackle the individual atoms.
Carbon should have 4, has 5 attached, formal charge = -1
Oxygen should have 6, has 5 attached, formal charge = +1
+1 and -1 cancel for a net formal charge of zero
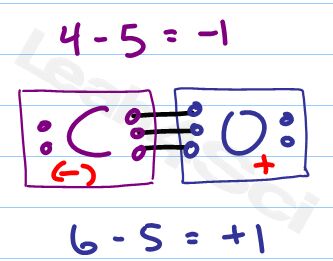
What do you think? Do you still find formal charge as tedious and daunting as you did when you first learned it? Hopefully the shortcut ‘should – has’ given you the confidence to simply stare at a molecule and come up with instant formal charges.
Or, are you needing more practice with Lewis Structures, Shape, Hybridization and more? Watch below!



Thank you soooo much…
Its so helpful!!! Thank you
Thank you Leah, you’ve been so helpful!!!
Literally awsum trick for solving formal charge in fraction of seconds…thank you
Amazing. Thank you very much for your great help. Nobody ever taught me that. It is an excellent time saver. Please keep on posting new shortcuts for different topics if you have.
Namaste Mam,
Thanks for your help, it’s easy to understand now.
i got it just now by watching it several times
thanks
Why don’t they teach this at school if it’s such a good equation?
Why are u teaching things that rnt in the curriculum
How do we treat a coordinate covalent bond?
What if some charge is present on compound
I mean lyk if compound is HClO4–??
What about this negative charge?
Very helpful ..thanx a lot
Wow… I never thought that I could easily calculate the formal charge without even remembering the formula.Superb trick !!!
I love you so so much.. But I, have to take entry test for the medical field.. We are given only 42seconds to solve the question and to fill up the circle.. Moreover, calculator is also not allowed in the test centre. I need short cut methods to calculate no of moles, no of atoms, no of ions and charges on them, volume of gas from the given mass or mass from given volume or given no of particles .also formulae to calculate tackle with mass to mass, mass to volume, volume to mass,no of particles to volume…. Similarly, molarity, molality, mole fraction, percentage w/w,w/v,v/w,v/v, and out of them find out molarity or molality etc…
Thank you so much for this excellent trick! You made it so easy!!
Thank uu it is very easy …..
Hi leah thank u so much for the tricks! 🙂 was wondering how then, would we know when to use dative bonding?
i want to know formal charge for O3
Thank you for this kind of help..
Could you discuss question I want to send on …?swap method
Please write about the Lewis structure of NO2—
thanks leah for your wonderful technique
When I first come to your site and read the tutorial,it was large and I was feeling boring but after reading it with concentration, I got the trick and It got worked.Really Very Helpful Trick.Kindly update some more tricks.
Regards,
Sagar Gupta
Thank you so much! This literally saved my life for the MCAT Gen Chem and Organic Chem sections! I struggled so much previously using the old equation and spent way too much time trying to go through the equation. This was so helpful!
Leah kindly do share more shortcuts.
Leah you helped me a lot after a long struggle in organic chemistry. You’re superb. Thanks a lot
Formal charge concept was bouncing over my head and now it’s over.Thank you for this easy trick.
You’re welcome!
leah madam namaste. i am so glad that u are highly knowledgable and i found no teacher as u in india where i study in a coaching institute to clear entrance exam for under graduation in mbbs. thank u leah maa’m. one day my success is sure in which u have a bit worthy role.
namaste leah madam. i have found no teacher as u in india where i am studying in a coaching institute to clear an entrance exam for undergraduation in mbbs. i hope that with ur simple n easy tricks i will be strong n bold enogh to solve the questions easily and get a good rank. thank u madam, u are so knowledgable . god will be always with u . keep on teaching like this .
Formal charge of carbon in benzene
Leah, can u pls find formal chrg of P in H3PO4, cz by ur formula the answer is zero by in my book it’s given +1..
Thnx
This really would have saved me some time on my last quiz…. I had no idea about this. Very cool.
Wow Leah, I didn’t know about this quick, short and time saving method. Thank you for such good work
Glad you found this helpful!
This should – has formula is great. But i’m having some problems with NO3-. In NO3-, one oxygen atom is bonded with nitrogen atom – one sigma and one pie bond. But there is a coordinate bond between N and the other O atom. so the formal charge on O should be= 6-(6+2). It should be -2. So how come it is -1? Can you clear my confusion pls.
You should only have 2 numbers in your equation NOT 3. Oxygen would be 6 – 7 for -1 or 6-6 = 0 for the other
You have done a fantastic job! Thanks a lot for all these, You are amazing…
Thanks Shubham
Formal charge was frustrating me for a long time,but after this “should-has” technique I feel like a burden is off my head. Thank you very much for your fantastic trick.
You’re very welcome. I felt the same way but now it’s second nature to me. Glad you feel the same way
The reason it’s normally not taught this way is that this just leads to a different confusion. The students response is “but I thought we need octets. Why does carbon only have 5 electrons in CO?”
I don’t understand your confusion. Carbon has 8 electrons in CO 5 attached and 3 more in the bonds for a total of 8
so one atom can be negative and the other positive, such as in (CH3)3NO. what would the overall charge be on that compound?
To find the overall or net charge, simply add up the individual formal charges.
This is very helpful! Thanks!
You’re welcome
Thanks now am going well with this series of tutorial but I need more different questions to make me practise on lewis structures and formal charge as well.
I agree Ally. I’m planning to add a quiz to this post down the line. For now refer to your textbook and look for as many problems as you can find to drive this topic home.
How would you work that out for N NH2 ? Suppose I take 5 electrons as ‘should’ and 1 electron from each Hydrogen which makes ‘Has’ 7. So, the formal charge on Nitrogen becomes -2 but it should be -1. Please tell me what I am missing?
I’m not sure I understand your question. Is your molecule N2H2? Or the N in NH2, and if so, first give everything their complete octet. Then determine that nitrogen should have five, hydrogen should have one, that’s twelve total. Count how many you have around each. Twelve will include bonds. But whatever is touching directly is considered in your formal charge calculation.
I’ve never seen a trivalent oxygen …how do u explain this?
Sheila: Oxygen with 3 bonds is very common in both organic and inorganic molecules. Think about ozone and hydronium, and so many more
Please find me the Bond order of ozone???| Thanks!
Wow! That’s a cool method!! Its so easy to find formal charge now…Thank you so much….does that trick work for all molecules without a single exeption? If so please do let me know.