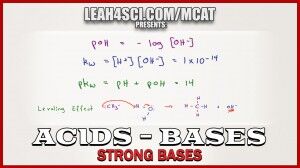 Acid base calculations get trickier when dealing with bases since most MCAT style questions will ask about pH instead of pOH. This means you have to be comfortable calculating both OH- and H+ concentrations to find pOH and pH values.
Acid base calculations get trickier when dealing with bases since most MCAT style questions will ask about pH instead of pOH. This means you have to be comfortable calculating both OH- and H+ concentrations to find pOH and pH values.
This video breaks it down with a few examples.
(click to watch on YouTube. Video transcript coming soon)
See Previous Video: pH Calculation for Strong Acids in MCAT Chemistry
See Next Video: pH and ka calculations for Weak Acids in MCAT Chemistry
This is video 3 in the MCAT Acid Base series. Catch the entire series HERE



For strong bases, why can’t we say 0.05 OH as Ca(OH)2 is a strong base (complete dissociation). Why we have to find that by using equation?