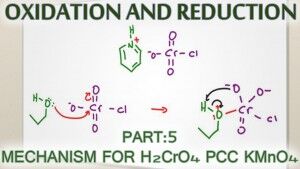
 Oxidation of alcohols can be carried out by a variety of reagents.
Oxidation of alcohols can be carried out by a variety of reagents.
The most common mechanisms you'll study in your organic chemistry course involve Chromic Acid H2CrO4, Pyridinium Chlorochromate PCC, and Potassium Permanganate KMnO4.
The video below shows you how each of these mechanisms will react with primary alcohols to form an aldehyde or carboxylic acid, and a secondary alcohol to form a ketone.
(Watch on YouTube: Alcohol Oxidation Mechanism. Click cc on the bottom right for video transcript)
<–Watch Previous Video: Oxidation of Alcohols to Aldehyde Ketone and Carboxylic Acid
–>Watch Next Video: Carbonyl Reduction using NaBH4
This is video 5 in the Orgo Oxidation/Reduction Series. Click for complete series
Ready to test your redox skills? Try the Redox Practice Quiz and follow along with the Redox Cheat Sheet



Great explanation!
I have a small doubt- In the Chromic Acid oxidation mechanism, when the ketone (or acid) forms, an electron gets kicked off onto the chromium (from oxygen) and the Chromium complex breaks off. What happens after to the Chromium after this? Does it get a negative charge?
Thanks in advance…
I like all of your video tutorials. Thank you very much for such a great work.
thanks a lot LEAH
You have taught me so much…thanks …please don’t you have an app for iPhones ?
Thank you
You’re welcome