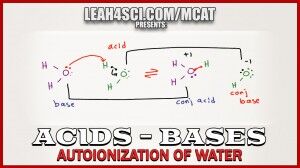
 Water is a fascinating molecule to study, especially in reference to acid base chemistry. Both amphoteric and amphiprotic (explained in video) it will undergo ionization to yield both an acid and a base.
Water is a fascinating molecule to study, especially in reference to acid base chemistry. Both amphoteric and amphiprotic (explained in video) it will undergo ionization to yield both an acid and a base.
This video breaks it down and covers important related calculations required for the MCAT.
(click to watch on YouTube. Read Video transcript here)
Resources Mentioned in the Video:
- Formal charge shortcut
- Solving Exponents/Square roots without a calculator
- Negative log shortcut without a calculator
See Previous Video: pH pOH ka kb Calculations for Weak Bases
See Next Video: pH/pOH Wheel: converting between pH pOH H+ OH- kw and pKw
This is video 6 in the MCAT Acid Base series. Catch the entire series HERE


