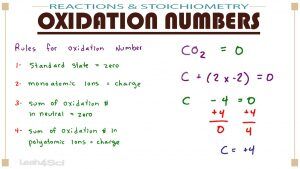
 Oxidation numbers can be confusing, unless you think of it as charge (real or imaginary) associated with each specific atom in a complex molecule or polyatomic ion.
Oxidation numbers can be confusing, unless you think of it as charge (real or imaginary) associated with each specific atom in a complex molecule or polyatomic ion.
Calculating Oxidation Numbers Video 1 – Oxidation Numbers
This video shows you how to find and calculate oxidation numbers when given simple and complex molecules or ions.
(Watch on YouTube: Oxidation Numbers. Click CC for transcription.)
Links & Resources Mentioned In This Video:
Calculating Oxidation Numbers Video 2 – Oxidation Numbers
Oxidation and reduction cannot happen on their own. When one atom loses electrons, another atom has to gain these electrons.
This video walks you through redox pairs using the mnemonic LEO the lion says GER to help you understand oxidizing/reducing agents. You’ll also learn how to recognize (rather than memorize) common oxidizing and reducing agents.
If you're looking to understand how assigning oxidation numbers can help you in studying things like the Galvanic/Voltaic or Electrolytic Cell, make sure to take a look at my Electrochemistry series.
(Watch on YouTube: Redox and Agents. Click CC for transcription.)
Links & Resources Mentioned In This Video:
<– Watch Previous Video: Density
–> Watch Next Video: Oxidation Reduction


