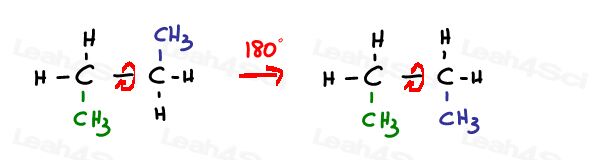 Your organic chemistry course will cover many different types of isomers.
Your organic chemistry course will cover many different types of isomers.
Isomers have the same molecular formula but something about them is different.
Geometric isomers, a type of stereoisomer, differ in their geometry or shape. This happens when substituents are LOCKED in a specific relationship to each other.
I say locked because, unlike conformational isomers in Newman Projections, you can’t simply rotate the molecule to change the relationship between substituents.
In this tutorial, we’ll look at alkene geometric isomers including cis trans and E Z.
Cis/Trans Isomerism
Cis/Trans isomerism is typically seen with substituents on either side of the alkene double bond.
How does this happen?
Alkene double bonds occur between sp2 hybridized carbon atoms. Recall: sp2 hybrids have a trigonal-planar or ‘flat’ geometry.
(Not comfortable with this? Review sp2 Hybridization.)
But it’s not the hybrid we’re looking at.
Instead, it's the un-hybridized p-orbital that forms a SECOND bond between the 2 carbon atoms.
An sp3 hybridized single or simga bond is free to rotate.
Sp2 pi bonds are locked in place.
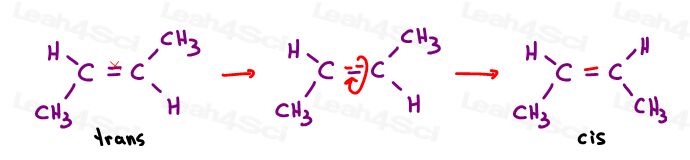
The only way to rotate this bond is to break the double bond, rotate, and reform the double bond –which is typically not observed.
In fact, this requires high energy, as you’ll see in your Diels Alder reactions later on.
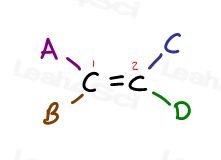 Take a look at the following generic alkene and it’s 4 substituents:
Take a look at the following generic alkene and it’s 4 substituents:
Carbon 1 has substituents A and B; Carbon 2 has substituents C and D.
But notice specifically how A is on the same side as C, and B is on the same side as D.
The only way to bring A next to D is to break the pi bond, rotate the molecule, and reform the pi bond. Otherwise A is locked in place near C, and B is locked in place near D.
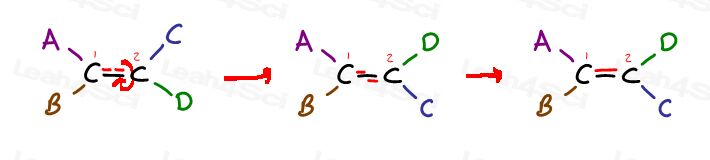
Cis vs Trans Alkenes
Let’s take a look at 2 versions of 2-butene: 2-butene is a 4-carbon chain with a double bond between carbons 2 and 3.
So, we can draw this incorrectly, as a linear molecule:
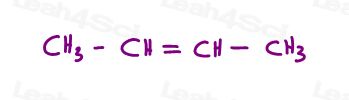
Or, draw each sp2 carbon at a 120 degree bond angle. This gives me the option of placing both methyl groups up, down, or one up and one down.
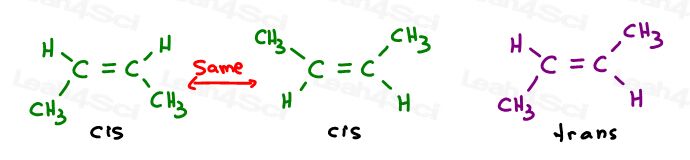
The first two are actually the same: both cis. You see, I can flip the molecule and make the first superimpose (overlap) the second without breaking any bonds.
The third is unique. The only way to superimpose the third is to break the double bond.
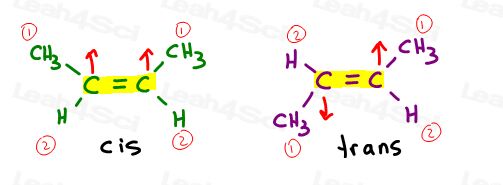
Cis Alkenes
I like to think of cis as ‘sisters’. They are together on the same side.
Cis alkenes have substituents on the same side of the double bond.
Trans Alkenes
I like to think of trans substituents as having ‘transferred away from each other.’ Putting them on opposite sides.
Trans alkenes have their substituents on opposite sides.
Naming Cis/Trans Alkenes:
Once you’ve identified cis/trans alkenes, naming them is fairly simple.
1) First, name the alkene using the tutorial linked below.
2) Then, simply add ‘cis’ or ‘trans’ in front of the name.
Take the 2 geometric isomers of 2-butene:
Their proper names are as follows:
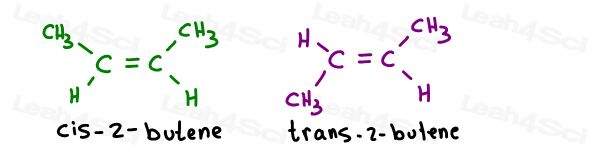
When there is only one pi bond, you don’t have to specify which carbon is cis or trans since. It’s self-understood.
When you have more than one double bond on the molecule, you must specify which is cis and which is trans.
Take this molecule for example: 2,5-octadiene
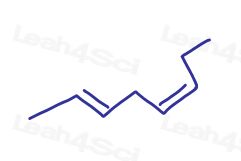
This molecule has 2 pi bonds. One cis and one trans.
Since there is more than one pi bond, you have to specify which pi bond is cis and which is trans.
Alkene stability
Not all isomers have the same stability.
It’s all about stability – in organic chemistry or science in general.

Trans alkenes are MORE STABLE than their cis counterparts.
This is more apparent with larger substituents.
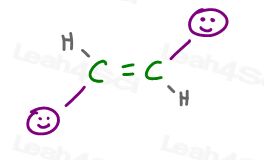 Trans Alkenes
Trans Alkenes
In a trans alkene, the substituents are facing away from each other.
They don’t ‘get in each other’s faces’ and therefore, don’t mind the other groups.
Cis Alkenes
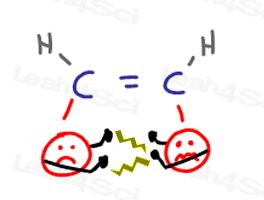 Cis alkene substituents are close together and will ‘get in each others faces.’ This causes ‘arguments’ when one group invades the other’s personal space.
Cis alkene substituents are close together and will ‘get in each others faces.’ This causes ‘arguments’ when one group invades the other’s personal space.
When the groups try to move away from each other, they cause strain on the molecule.
All of this leads to an unhappy and higher energy cis conformation.
Cis & Trans on Cyclic Compounds
Ring structures or cyclic compounds can also exhibit cis/trans isomerism without the presence of a pi bond.
Remember, substituents will be cis and trans if they are locked in place. Pi bonds are one way to lock them in place. Rings are another matter.
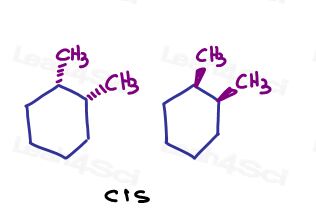
For example, in 1,2-dimethylcyclohexane, I can show both substituents going into the page or both going out of the page.
Since they’re pointing in the same direction, they are cis to each other.
If I show one going into the page and one going out of the page. They are trans to each other.
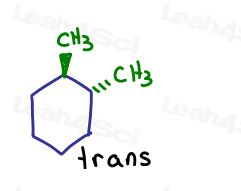
Even though the carbons are sp3 and sigma bound to each other, the molecule itself cannot rotate because of the ring structure. Locked.
The only way to turn cis-1,2-dimethylcyclohexane into trans-1,2-dimethylcyclohexane, is to break open the ring, rotate, and reform the ring.
What if there’s more than one substituent on the sp2 carbon?
Till now, we’ve looked at molecules with just one substituent on either side of the sp2 pi bound carbon.
What happens if we have a pi bond with 2 different atoms or groups on the sp2 carbon?
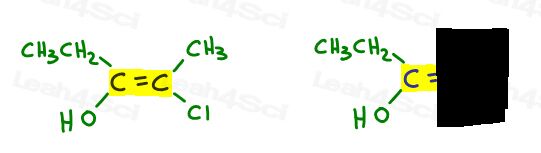
Take a look at 3-methyl-2-pentene:
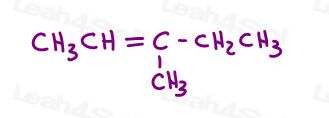
Here in line structure:
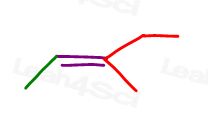
You can draw this molecule in 2 different ways. But will you compare the red methyl or red ethyl to the green methyl when choosing cis or trans?
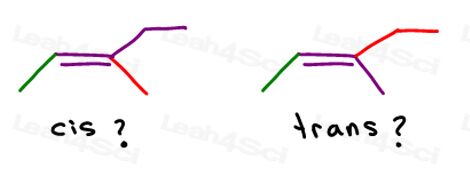
While some professors WILL teach you to compare the larger groups, the answer is that you CANNOT compare simply choose one for cis and trans.
Introducing the E Z Notation
When a pi bond has more than one substituent on each side, or contains non-carbon substituents, we’ll need a more advanced system for identifying geometric isomerism.
The E Z system requires ranking the groups on either side of the pi bond. We must determine if the higher priority groups are next to each other, Z (think cis), or away from each other, E (think trans).
But first, we have to learn how to rank groups using the Cahn-Ingold-Prelog notation.
The video below is from my chirality series, but teaches this concept in detail. Start watching from 0:52
Cahn-Ingold-Prelog in summary:
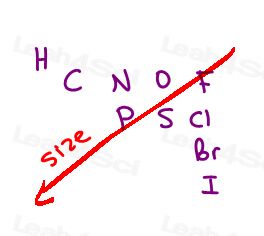
We’re ranking atoms based on their atomic number.
Not mass of the group, not size of the group.
The higher the atomic number of the atom directly attached, the higher the priority.
Here are the 10 most common atoms you’ll come across from high to low priority:
I > Br > Cl > S > P > F > O > N > C > H
Here’s My Simple Approach
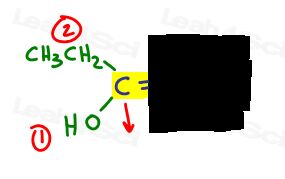
- Highlight your double bond, then look at just one half of the molecule at a time. Use your hand or another paper to cover the other half of the molecule.
- Determine which group is higher priority and put a number 1.
I like to draw an arrow perpendicular to the pi bond so I can clearly see if it’s up or down by comparison.
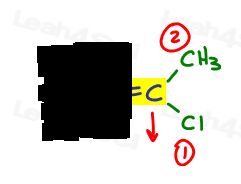
- Do the same thing for the other side.
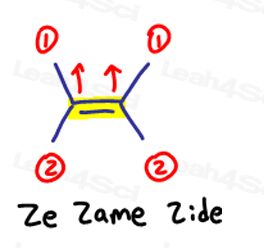
E is for Eeposite, Z is for Ze Zame Zide
If the two high priority groups are opposite to each other, think of them as being ‘eeposite’ to each other.
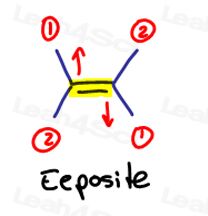 E is for Eeposite.
E is for Eeposite.
If the two high priority groups are on the the same side, or should I say on ‘Ze Zame Zide,' they are Z.
This applies to molecules that have more than just 1 carbon on either side of double bond.
Ze Zame Zide.
Let’s go back to the example above:
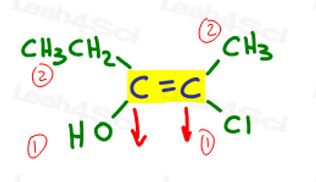
On the left, OH outranks ethyl since oxygen has a higher atomic number when compared to carbon. OH is #1 and points down.
On the right, Cl outranks methyl since chlorine has a higher atomic number when compared to carbon. Cl is #1 and points down.
Since both arrows point in the same direction (down), we conclude that the priority groups are on Ze Zame Zide making it Z.
2 Equal Priority Groups
Sometimes you’ll see a trick question where an sp2 carbon will have 2 of the exact same groups.
Since you cannot rank one over the other, there will be NO cis/trans or E/Z isomerism.
Here are 2 common examples:
1) A terminal pi bond
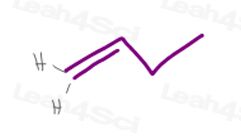
Carbon #1 in 1-butene has 2 hydrogen atoms.
Since H vs H have the same exact priority, this molecule will have no cis/trans or E/Z isomerism.
2) Same exact groups on the same sp2 pi bound carbon.

Carbon #2 in 2-methyl-2-butene has 2 CH3 groups.
One appears to be part of the parent chain, the second appears to be a methyl substituent.
However, when CH3 is compared to CH3 they rank exactly the same.
This molecule will have no cis/trans n/or E/Z isomerism.
Cis and Trans vs E and Z
If we go back to our cis/trans practice problems, such as cis and trans 2-butene, you’ll see that we can use the E/Z system here as well.
Carbon 2 and 3 each have a methyl group outranking a hydrogen atom. When they are cis, you get Z. When they are trans you get E.
A word of caution
You CAN use E/Z for cis/trans isomers, but you cannot use cis/trans for complex E/Z isomers as we’ve already shown above.
In Summary
Cis vs trans and E vs Z isomers are geometric isomers that occur when substituents are locked in position next to or opposite each other. This is seen in both double bonds for alkenes, and substituents on ring structures.
Cis alkenes are on the same size, trans alkenes are on opposite sides. When the substituents are more complicated use the more advanced E/Z notation after determining the relationship of high priority groups.