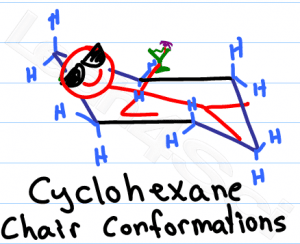
 Studying chair conformations is likely one of the trickiest visual topics in organic chemistry, perhaps second only to Fischer projections. Not only are you required to learn a 3-dimensional concept, but you also have to manipulate that 3-D molecule on 2-dimensional paper.
Studying chair conformations is likely one of the trickiest visual topics in organic chemistry, perhaps second only to Fischer projections. Not only are you required to learn a 3-dimensional concept, but you also have to manipulate that 3-D molecule on 2-dimensional paper.
The average orgo student is not an artist, making this topic even trickier. In this article I'll show you a few quick and simple tricks to help you easily draw the standard hexagon, chair conformations and ring flips for cyclohexane.
When it comes to exams, you won't be graded on how ‘pretty' your chair looks. Instead your professor will look for clarity and the ability to distinguish your axial and equatorial substituents.
Drawing the Cyclohexane Hexagon
If there's one thing you learn in organic chemistry – it's how to draw a hexagon. Many students try to draw the entire thing at once, but the poor hexagon tends to look drunk and wobbly.
In fact, if you're starting to feel like the class is one big art lesson, read here: Organic Chemistry or Art Class?
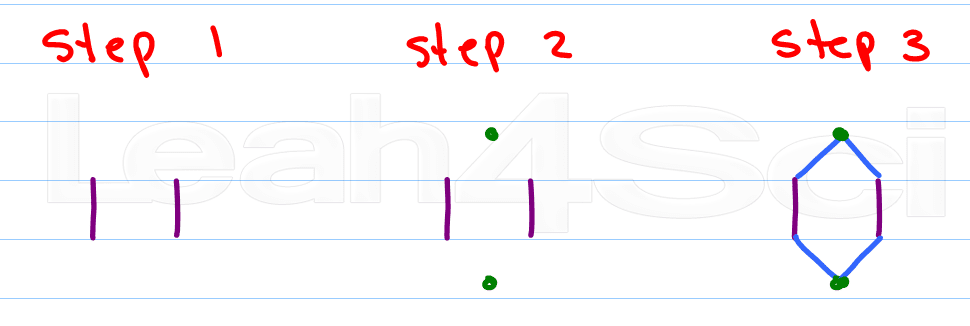
Here's my approach:
- Draw 2 parallel lines
- Place a dot above the upper opening and another below the lower opening
- Connect the dots
Pretty? Not especially
But clear? Absolutely!
And that's what really matters.
As you get better you can combine steps 2 and 3 by simply visualizing the location of the dots.
Drawing the Cyclohexane Chair Conformation
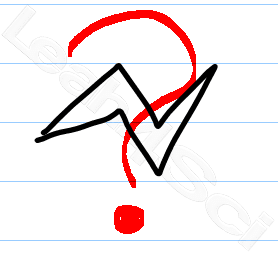 This is where the messiness and confusion arises. Most books will show a chair conformation slightly sideways, making it impossible to copy. Worse, it's really difficult to show which substituents are axial vs equatorial.
This is where the messiness and confusion arises. Most books will show a chair conformation slightly sideways, making it impossible to copy. Worse, it's really difficult to show which substituents are axial vs equatorial.
‘Bowties' as I like to call them, are ok for the computer generated chair conformation. Here is my simple version which my tutoring clients use on exams with great success.
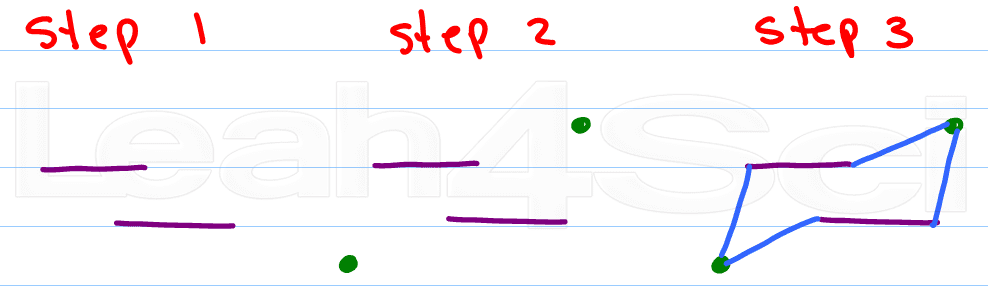
Step 1:
Draw 2 parallel lines slightly offset from each other. Top left or top right will give you alternate chairs.
Step 2:
Place a dot above the upper opening, and another below the lower opening
Step 3:
Connect the dots
Kinda sounds like the directions for drawing a cyclohexane, doesn't it?
But the chair confirmation doesn't stop here. It's important to understand how to position your substituents. And even more important to show what is axial and what is equatorial.
So let's move on:
Step 4:
Identify the ‘up tip' OR ‘down tip' of your chair conformation, and draw a straight line up (up tip) or down (down tip) parallel to the y-plane. This is your first axial substituent.
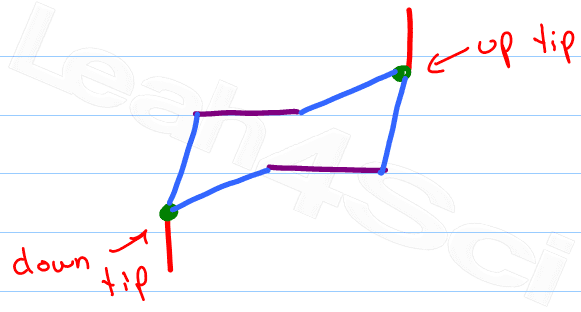
The chair conformation has alternating axial up, axial down… so once you have that single axial substituent move on to..
Step 5:
Alternate your axial substituents up and down all the way around your cyclohexane
Every carbon on the chair conformation has 1 substituent axial and the other equatorial. If axial is up equatorial is down, if equatorial is up then axial is down.
Step 6:
Pick any ONE carbon and locate its axial substituent. Draw the equatorial substituent up or down, the reverse of the axial substituent. But unlike the axial line, this one will start on the carbon and form a slight angle outward of the chair drawing. Do NOT draw these straight in the x or y plane.
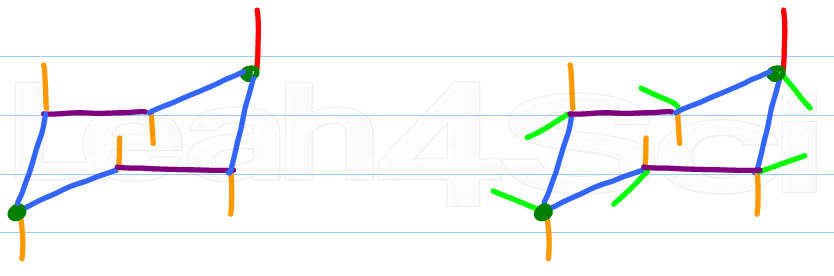
Adding substituents to your chair
This is my favorite part. I find students get so confused when they try to match the cyclohexane to the drawing.
I don't!
All I care about matching are the numbers.
Start with a blank chair conformation. Number the carbons in your cyclohexane and in your chair.
Clockwise or counterclockwise doesn't matter, as long as you use the same direction for both molecules.
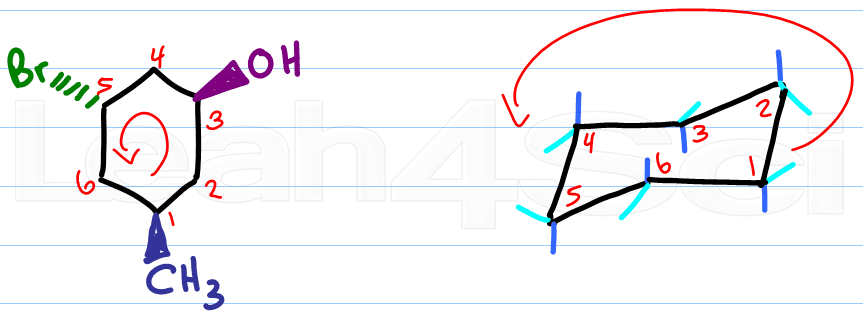
Then simply compare.
Identify the carbon number for the first substituent, if it's wedged add it to the up position.
If the substituent is dashed, add it to the down substituent (dashes down)
In using this trick you simply match the numbers and let your molecule quickly fall into place
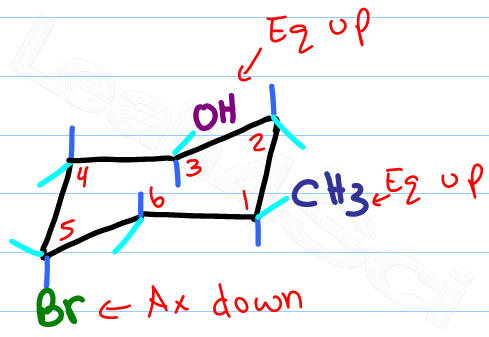
Drawing a Ring Flip
The logic and reasoning behind the chair conformation ring flip will be discussed in my upcoming chair conformations video series. (stay tuned) However, drawing the ring flip doesn't have to be as hard as students think.
Yes the flip happens when one molecule changes its conformation to another, but the key to drawing the flip successfully is to… Ignore the first chair!
Counter-intuitive, I know, but trust me this method works.
Once you have your first chair, determine if your parallel lines have upper right or upper left.
Draw another chair using the steps described above, but change the direction of your top line.
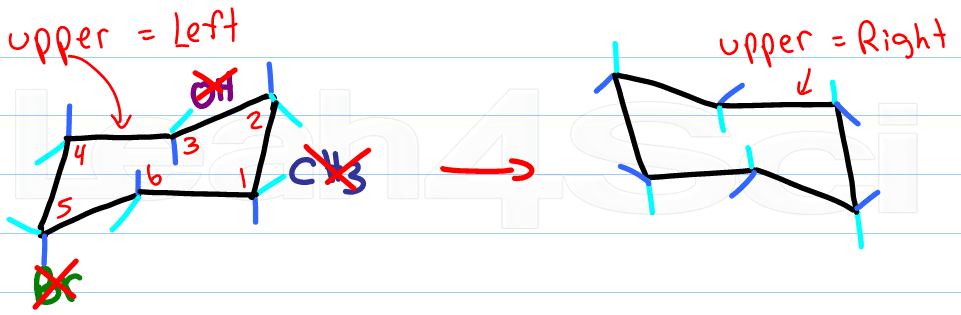
If your first chair has the upper line on the right, draw the second chair with the upper line left.
Ignore your first chair as you follow the rules for drawing and adding substituents.
Now for the fun part, determine the new location for the up-tip carbon by ‘pulling down' your ‘up-tip' or raising up your ‘down-tip' Number your new chair, and play ‘match the numbers'.
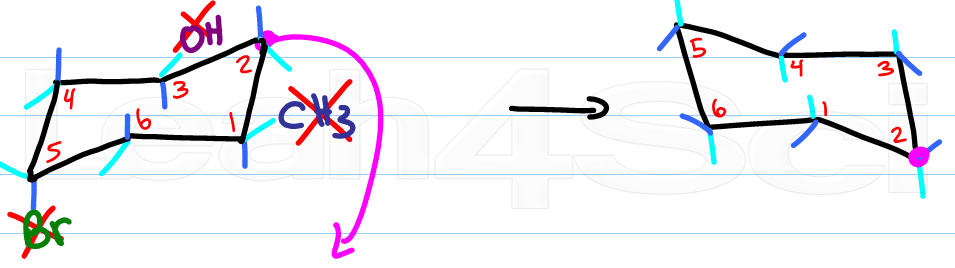
If there's a substituent up on carbon #1, it says up on carbon #1. If it's down it stays down.
The only thing that changes with your ring flip is the location of axial and equatorial.
Remember this:
Up stays up, down stays down
Axial becomes equatorial and equatorial becomes axial
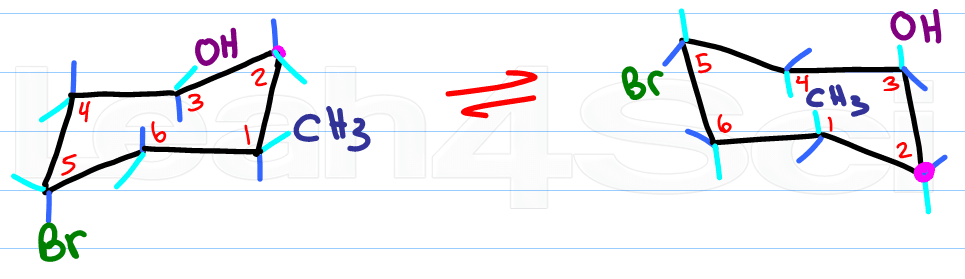
Hopefully you've been drawing chairs as you read this article. What do you think? does the idea of drawing chair conformations and ring flips still sound as scary as it used to? Let me know by leaving a comment below
Watch the video below for a complete demonstration of what we learned above.
Ready to tackle some chair conformations? Learn about their structure, stability, flips and more in the Cyclohexane Chair Conformations Tutorial Video Series.
Would a Model Kit help you? Watch How to Use Your Organic Chemistry Model Kit.


