 EAS Series: Video 3
EAS Series: Video 3
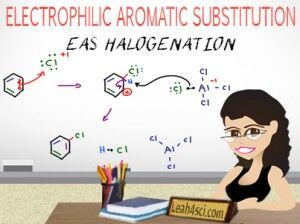
This video will start by showing you a quick comparison of alkene halogenation versus aromatic halogenation: bromination and chlorination. This video will show you the aromatic halogenation mechanism from the role of the Lewis Acid catalyst and formation of the super-electrophile, through the entire mechanism of adding halogen to benzene. Also shown is a break down of the reaction mechanism for aromatic halogenation using Cl2 with the Lewis acid catalyst AlCl3.
(Watch on YouTube: Halogenation. Click CC on bottom right for transcript.)
Links & Resources Mentioned In This Video:
<– Watch Previous Video: EAS Mechanism and Sigma Complex Resonance Video
–> Watch Next Video: EAS Aromatic Nitration Reaction and Mechanism Video


