 Intro to Orgo Series: Video 5
Intro to Orgo Series: Video 5
This tutorial video is designed to help new and incoming organic chemistry students build a solid foundation for organic chemistry.
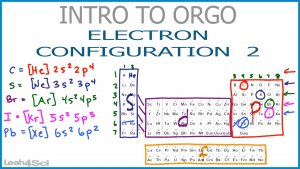
This video has additional examples of finding the electron configuration of larger atoms and the shortcut version using the noble gas kernel.
Video 4 (Electron Configuration Part 1) showed the introduction.
(Watch on YouTube: Configuration 2. Click cc on the bottom right for video transcription.)
<– Watch Previous Video: Electron Configuration Part 1
–> Watch Next Video: Lewis Dot Diagram & Octet Rule
This is Video 5 in the Intro to Orgo Video Series. Click HERE for the entire series.
Want to test your understanding? Try the Gen Chem Review for Orgo Quiz after watching the series!



Hi, in this video for Bromine’s shortened configuration you remove the 3d10 as it is in a lower energy shell however, in the quiz you say cobalt’s configuration is [Ar] 4s2 3d7 why is the 3d orbital included in the shortened version in this case but not for Bromine??? Thank you so much for your wonderful videos, they are keeping me sane!
I know carbon has 6 electrons, how come it looks like he has 8 electrons when you write its electron configuration? 1s2-2s2-2p4? Thanks.
The correct answer is 1s^2 2s^2 2p^2. Thanks for catching that error.
is it possible to write Noble gas configurations in an excited state? And how would you write normal excited state electron configurations?
Anything can be written in an excited state, simply bump the n-value of the excited electron
I’m confused with noble gas configuration of Carbon..??
Carbon is not a noble gas, however the kernal or internal shell can be related to the noble gas Helium
Ok, I understand that. But why is the electron configuration for carbon 1s2 2s2 2s4, instead of 1s2 2s2 2p2. Wouldn’t thwt make [He] 2s2 2p2 ?
Why didn’t you include the d orbital in the shortcut for the electron configuration?
The ‘d’ orbital is a high energy orbital, however it occurs at a lower level overall. This means that while it is identified later on, it’s actually in the shell below and therefor not part of the valence. I know this can be confusing, and so keep in mind this trick: Valence refers to the outermost s and p orbitals
i love ur videos …..It was really helpful !!!! thank u soooo much 🙂
Thank you Roshini, I’m glad you find them helpful