 Electrophilic Aromatic Substitution is one of the more exciting topics covered in organic chemistry. Some schools teach this in Orgo 1, others in Orgo 2. But it doesn't end there, this topic is often tested on the MCAT, DAT and similar – with a focus on your ability to understand and deduce mechanism intermediates and reaction products.
Electrophilic Aromatic Substitution is one of the more exciting topics covered in organic chemistry. Some schools teach this in Orgo 1, others in Orgo 2. But it doesn't end there, this topic is often tested on the MCAT, DAT and similar – with a focus on your ability to understand and deduce mechanism intermediates and reaction products.
My goal with this video series: to give you a detailed understanding of the reaction basics, without going into crazy (irrelevant) details, so that you can understand enough to really get it. Yet work through it fast enough on your quizzes and exams!
This topic assumes that you already have a solid foundation on aromaticity. Need a review? See my Aromaticity Tutorial.
In this type of reaction, aromatic hydrogen atoms are replaced by another atom (such as a halogen) or group of atoms such as a nitro, or acyl group. While EAS may sound a bit like the SN1/SN2 reaction, the mode of attack is very, very different.
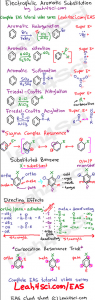
Click HERE to grab my EAS Cheat sheet: Summary of EAS reactions, resonance, and directing effects
Included in this series:
- Introduction to Electrophilic Aromatic Substitution
- EAS Mechanism + Sigma Complex Resonance
- Basic EAS reactions adding to Benzene:
- Aromatic – Halogenation, Nitration, Sulfonation
- Friedel-Crafts – Alkylation, Acylation, Comparison of Alkylation vs Acylation Reactions
- Directing Effects on Substituted and Multi-Substituted Benzene:
- Ortho/Meta/Para – ‘Monster' Trick Video, Shortcut for Substituted Benzene
- Activating Groups – Ortho/Para Directors
- Deactivating Groups – Meta Directors
- Halogens are exceptions – Deactivating Ortho/Para Directors
- Electrophilic Aromatic Substitution Cheat Sheet
Video 1 – Introduction to Electrophilic Aromatic Substitution
The EAS Intro video below gives you a detailed overview of the EAS reaction, along with a comparison to alkene addition reactions and the need for a Super-Electrophile
Video 2 – EAS Mechanism + Sigma Complex Resonance
Since benzene rings are aromatic, they are less than thrilled to ‘break open' and undergo an EAS reaction. However, the intermediate does have some stability due to its ability to resonate the 2 remaining pi bonds on the sigma complex.
◊ Basic EAS reactions adding to Benzene ◊
While many of your exam questions will focus on adding groups to a monosubstituted or disubstituted benzene, it's important that you first get a solid understanding of the basics. The next few videos will take you through the most common EAS reactions, focusing on the mechanism for the super-electrophile creation as well as the actual addition to benzene. The most common EAS reactions you will cover are as follows:
Video 3 – EAS Aromatic Halogenation 
Adding Cl or Br to the benzene ring.
This video shows you the aromatic halogenation mechanism from the role of the Lewis Acid catalyst and formation of the super-electrophile, through the entire mechanism of adding halogen to benzene.
Video 4 – EAS Aromatic Nitration
Adding a nitro group (NO2) to the benzene ring.
This video shows you the mechanism for the formation of Nitronium – the super electrophile that is attacked by benzene in the nitration reaction.
Video 5 – EAS Aromatic Sulfonation 
Adding SO3H to the benzene ring.
The video below shows the complete mechanism from creating a super electrophile (SO3) to sigma complex and reforming the benzene ring.
Video 6 – EAS Friedel-Crafts Alkylation
 Adding an alkyl group to the benzene ring.
Adding an alkyl group to the benzene ring.
This video shows you the mechanism for the formation of carbocation in a Lewis Acid as the super electrophile that is attacked by benzene in the Friedel-Crafts Alkylation reaction.
Video 7 – EAS Friedel-Crafts Acylation
Adding an acyl group to the benzene ring.
This video shows you the mechanism for the formation of acylium ion with a Lewis Acid catalyst to form the super electrophile that is attacked by benzene in the Friedel-Crafts Acylation reaction.
Video 8 – EAS Friedel-Crafts Alkylation vs Acylation 
Comparison and limitations.
This video shows you a comparison of the FC alkylation and acylation reactions, including the limitations of FC alkylation, and how to convert an acylation product to the reduced alkyl version.
◊ Directing Effects On Substituted and Multi-Substituted Benzene ◊
When it comes to a substituted benzene, the chemistry and nature of the existing substituent (or substituents) will determine not only where the next group adds onto the ring, but also if the group is allowed to add at all.
Substituents on benzene will fall into 2 main categories – activators and deactivators. Another way to look at them is to see if they direct the incoming groups to the Ortho, Meta, or Para positions. To learn more, read about Ortho, Meta, Para Directing Effects in EAS reactions here.
Video 9 – Ortho Meta and Para Disubstituted Benzene Monster Trick 
Confused about Ortho, Meta, and Para? Let the monster in the video below help you remember! This video shows my fun trick for easily recognizing ortho, meta, and para substituents on a disubstituted benzene.
Video 10 – Resonance and Trick for Ortho Meta and Para Addition Intermediates 
The key to mastering directing effects of activating and deactivating groups lies in understanding the nature of the sigma complex resonance intermediates. There's the long way, drawing resonance structures, and the short way using my awesome shortcut.
Video 11 – Ortho Para Directing Groups 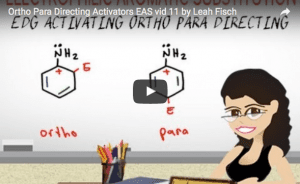
Activating Groups are Ortho/Para Directors.
Now that you've mastered resonance, let's take a look at the directing effects of activating and deactivating groups.
This video takes you through the logic and structure of the ortho and para directing activating groups.
Video 12 – Meta Direction Groups Video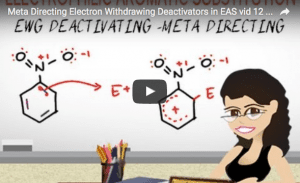
Deactivating Groups are Meta Directors.
This video shows you the nature of deactivating groups as meta directors through through logic and structure to help you avoid memorization.
Video 13 – Halogen Exception Ortho Para Deactivators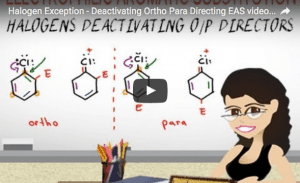
Halogen Exception!
This video shows you the unique directing effects of halogens on substituted benzene. Learn why they are ortho para directing yet still deactivate the benzene ring towards electrophilic aromatic substitution.
Electrophilic Aromatic Substitution Cheat Sheet
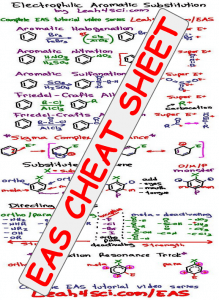 Studying the EAS reactions may not be enough.
Studying the EAS reactions may not be enough.
I've put together a comprehensive cheat sheet to summarize the major EAS reactions, sigma complex mechanism, substituted benzene – ortho meta para, directing groups and their directing effects.
This also holds a quick trick for directing group intermediate resonance.
Click the cheat sheet thumbnail for information on downloading the full version FREE!
<– Back to the Organic Chemistry Syllabus Companion
to see what to study next!


