 Alkene Reactions Series: Video 4
Alkene Reactions Series: Video 4
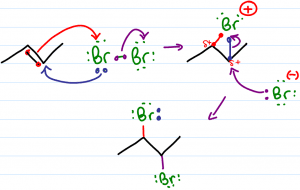
Halogenation of alkenes is the reaction in which a double bond is broken and replaced by a vicinal dihalide – 2 halogen atoms added to neighboring carbons. This reaction follows a pattern of anti addition.
The goal of this video is to help you understand rather than memorize concepts related to the halogenation mechanism. This video addresses dihalide polarization, bromonium/chloronium bridge formation, addition to cyclic and asymmetrical starting alkenes.
(Watch on YouTube: Halogenation. Click cc on the bottom right for video transcription.)
<– Watch Previous Video: Hydrohalogenation of Alkenes
–> Watch Next Video: Halohydrin formation
This is Video 4 in the Alkene Reaction Mechanisms Video Series. Click HERE for the entire series.
Ready to test your skills? Try the Alkene Reactions Practice Quiz after watching the series!


