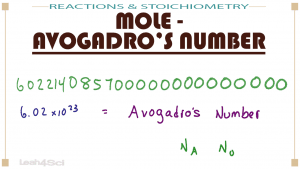 Moles and Avogadro's number are big! And they come across as overwhelming. But YOU can master them!
Moles and Avogadro's number are big! And they come across as overwhelming. But YOU can master them!
This video covers the definition of Moles, with an illustration showing the logic of moles in general chemistry. You'll also see how to easily and quickly convert from and to them on the MCAT.
You'll see conversions including atoms, AMU, grams, and moles. Let's knock this out on your MCAT Content Category List!
(Watch on YouTube: Mole and Avogadro's #. Click CC for transcription.)
Links & Resources Mentioned In This Video:
<– Watch Previous Video: Percent Mass by Composition
–> Watch Next Video: Density


