 Molecular Orbital theory is a topic that comes up in general chemistry in relation to bond formation and bonding energy.
Molecular Orbital theory is a topic that comes up in general chemistry in relation to bond formation and bonding energy.
At the organic chemistry level, we’re less concerned with the nitty-gritty quantum physics and crazy math and more interested in making just enough sense of the information to be able to apply what we know to reactions and mechanisms.
MO theory typically comes up when studying reactions of conjugated systems, especially when asked to identify HOMO and LUMO molecular orbitals for molecules in Diels-Alder and other pericyclic reactions.
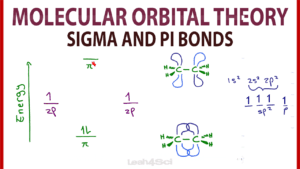
Video 1: Molecular Orbital Theory for Sigma and Pi Bonds
This video provides you with a logic-based overview of MO theory with simple take-aways to focus on at the organic chemistry level.
Video 2: HOMO and LUMO for Conjugated Systems
This video shows you how to draw the different molecular orbitals for a conjugated system, how to make sense of the logic without the crazy math, and how to quickly and easily identify HOMO and LUMO molecular orbitals.


