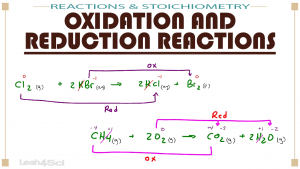
 Oxidation and Reduction reactions happen all the time in a living system and thus are very likely to show up on the MCAT. This video walks you through oxidation and reduction reactions, how to figure out the half reactions, and how to easily recognize (+ trick) what gets oxidized and what gets reduced. You’ll also see how to identify redox species in Disproportionation, Single Replacement, Double Replacement, Combination/Synthesis and Combustion reactions.
Oxidation and Reduction reactions happen all the time in a living system and thus are very likely to show up on the MCAT. This video walks you through oxidation and reduction reactions, how to figure out the half reactions, and how to easily recognize (+ trick) what gets oxidized and what gets reduced. You’ll also see how to identify redox species in Disproportionation, Single Replacement, Double Replacement, Combination/Synthesis and Combustion reactions.
If you're looking to see how redox reactions come into play in the Galvanic/Voltaic or Electrolytic cell, make sure to visit my Electrochemistry series.
(Watch on YouTube: Oxidation Reduction. Click CC for transcription.)
<– Watch Previous Video: Oxidation Numbers
–> Watch Next Video: Chemical Equations


