 As an organic chemistry tutor, one of the first and more complicated topics that my students need help with is how to quickly recognize primary, secondary and tertiary carbon atoms within a molecule. There are a number of ways to do this.
As an organic chemistry tutor, one of the first and more complicated topics that my students need help with is how to quickly recognize primary, secondary and tertiary carbon atoms within a molecule. There are a number of ways to do this.
I’ll explain them then show you a trick you can apply to get your answer quickly on an exam. I call it the pencil trick.
After reading, be sure to watch the step-by-step Video Tutorial at the bottom of this page.
Overview of Primary Secondary and Tertiary Atoms
Primary, secondary or tertiary carbon refers to the number of carbons directly attached to the carbon in question.
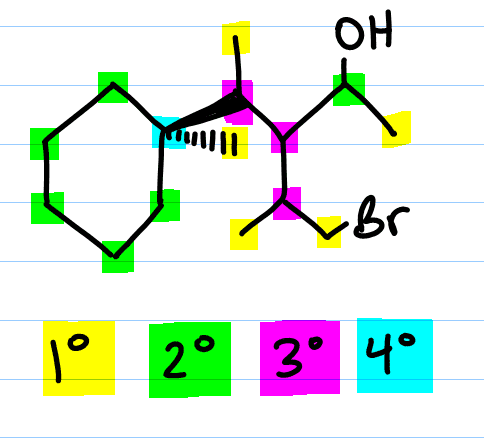 In other words:
In other words:
- A primary carbon can be written as 1° (#1 with a degree symbol) has one carbon attached to this carbon atom.
- A secondary carbon written as 2° (#2 with a degree symbol) is a carbon attached to two other carbons.
- A tertiary carbon written as 3° (#3 with a degree symbol) is a carbon attached to three other carbons.
- And a quaternary carbon written as 4° (#4 with a degree symbol) is a carbon attached to four other carbons.
I want you to recognize that the quaternary carbon is attached ONLY to carbon. This is because having four bonds to carbon means that carbon already has its complete octet. But primary, secondary and tertiary carbons will have other atoms attached. These atoms can include hydrogen, halogens (F, Cl, Br, I), oxygen, nitrogen, and so on.
Degree of Substitution
Degree of substitution means how many carbons are attached to the carbon atom in question. The answer would be something like primary, secondary or tertiary depending on what you have. The quickest way to recognize the degree of substitution is to look at the carbon in question and count how many carbon atoms stem from it.
When you have a molecule written in Lewis Structure it’s easy to see but when the molecule is written in line structure it can get tricky.
This is Where The Pencil Trick Comes In To Play
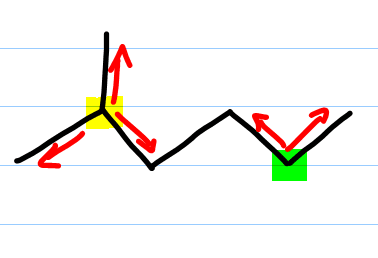
Here’s how to quickly recognize if a carbon atom is primary, secondary, tertiary or quaternary using the pencil trick. Identify the carbon in question and place your pencil on that carbon atom. Then examine how many lines emanate from the carbon atom that attach to another carbon—meaning the line does not lead to a hydrogen, oxygen or halogen. The number of lines that stem from that carbon represents the degree of substitution. Look back at the image above.
On the image I highlighted one carbon atom in yellow which, if you put your pencil on it, has three lines emanating from it making it a tertiary carbon. If you put your pencil on the other carbon atom, highlighted in green, you’ll see two lines stemming from it, making this a secondary carbon.
Alcohol and Halogen Substitution
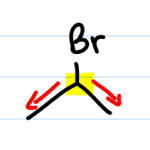
When referring to the degree of substitution of a halogen or an alcohol, you don’t look at the halogen or alcohol itself but rather at the carbon holding it.
So for example, if you look at bromine in the molecule drawn here, you’ll see that bromine is attached to one carbon. But this bromine is not a primary halogen even though it’s attached to one carbon. You must look at the carbon instead. Do the pencil trick and see that this carbon is attached to two other carbons. Since the carbon is secondary, so is the bromine.
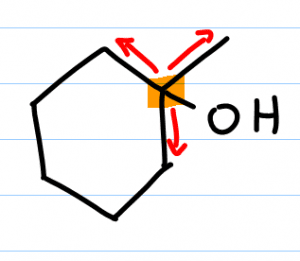
The same thing applies for alcohol. For example, if the alcohol is on the tertiary carbon then the alcohol is considered tertiary; not because it’s attached to one carbon but because the carbon holding the alcohol is attached to three other carbons.
Amines (Nitrogen) Are Classified Differently
Degree of substitution for amines is slightly different than alcohols and halogens because in this case we’re actually looking at the nitrogen atom.
A primary amine is one that has the nitrogen or an NH2 group directly attached to one carbon. We don’t care if this is a primary, secondary or tertiary carbon because it’s the nitrogen that we’re examining.
A secondary amine is an NH group attached to two other carbons.
A tertiary amine is a nitrogen attached to three other carbons.
Sometimes you'll see an ammonium salt, which is a positive nitrogen attached to four different carbons.
The reason I said NH2, NH and N for primary, secondary and tertiary is because we’re assuming our amines are neutral. If nitrogen can have a total of three bonds and one lone pair, we expect that when nitrogen is bound to one carbon, it will have two hydrogens.
When nitrogen is attached to two carbons it will have one hydrogen.
When nitrogen is attached to three carbons it will not have hydrogen.
That’s why ammonium is the different one, the positive nitrogen has 4 bonds and no lone pairs.
You can actually apply the pencil trick to nitrogen as well. Just put your pencil on nitrogen and see how many carbons stem from it.
(Watch on YouTube: Pencil Trick. Click cc for transcription.)


