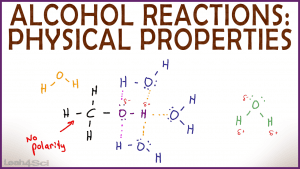 The key to understanding physical properties of alcohol, including hydrogen bonding solubility and boiling point is to understand the structure and charge distribution within the alcohol functional group.
The key to understanding physical properties of alcohol, including hydrogen bonding solubility and boiling point is to understand the structure and charge distribution within the alcohol functional group.
This video will show you the behind-the-scenes intermolecular forces at work that affect alcohol's ability to hydrogen bond for solubility in water, miscibility, and boiling point trends.
You'll also see how alcohol chain length and branching impact these properties.
(Watch on YouTube: Alcohol Properties. Click cc for transcription.)
Links & Resources Mentioned In This Video:
- Naming Alcohols Video Tutorial
- Bonding Review: Ionic, Polar Covalent, and Non-Polar Covalent
<– Watch Previous Video: Introduction to Alcohol Reactions
–> Watch Next Video: (Coming Soon!)
This is Video 2 in the Alcohol Reactions Video Series. Click HERE for the entire series.
Alcohol Practice Quiz and Cheat Sheet Coming soon!


