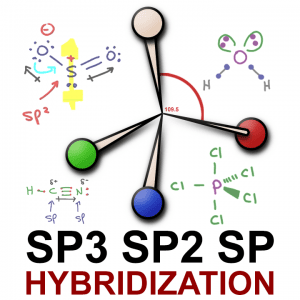
 Sp³, sp² and sp hybridization, or the mixing of s and p orbitals which allows us to create sigma and pi bonds, is a topic we usually think we understand, only to get confused when it reappears in organic chemistry molecules and reactions.
Sp³, sp² and sp hybridization, or the mixing of s and p orbitals which allows us to create sigma and pi bonds, is a topic we usually think we understand, only to get confused when it reappears in organic chemistry molecules and reactions.
When I took general chemistry, I simply memorized a chart of geometries and bond angles, and I kinda/sorta understood what was going on. But it wasn’t until I started thinking of it in a different way, as I’ll explain below, that I finally and truly understood.
In this article, we’ll cover the following:
- WHY we need Hybridization
- HOW Hybridization occurs
- VSEPR Theory
- Sp³ Hybridization, Bond Angle and Geometry (includes video)
- Molecular vs. Electronic Geometry
- Sp² Hybridization, Bond Angle and Geometry (includes video)
- Sp Hybridization, Bond Angle and Geometry
- Hybridization Shortcut
- Sp³d and sp³d² Hybridization – An Overview
So let’s dig in.
In addition to undergrad organic chemistry, this topic is critical for exams like the MCAT, GAMSAT, DAT and more.
Why do we need hybridization?
Molecules are everywhere! How do they form?
Simply put, molecules are made up of connected atoms,
Atoms are connected through different types of bonds,
With covalent bonds being the strongest and most prevalent.
In order to create a covalent bond (video), each participating atom must have an orbital ‘opening’ (think: an empty space) to receive and interact with the other atom's electrons.
Let’s take a quick detour to review electron configuration with a focus on valence electrons, as they are the ones that actually participate in the bond.
Feeling rusty? Click to review my Electron Configuration + Shortcut videos.

Take hydrogen.
It has a single electron in the 1s orbital. It requires just one more electron to be full.
This leaves an opening for one single bond to form.
Great for adding another hydrogen, not so great for building a large complex molecule.
Now, consider carbon.
It’s no coincidence that carbon is the central atom in all of our body’s macromolecules.
Proteins, amino acids, nucleic acids– they all have carbon at the center.
Why? Because carbon is capable of making 4 bonds.
That’s a lot by chemistry standards!
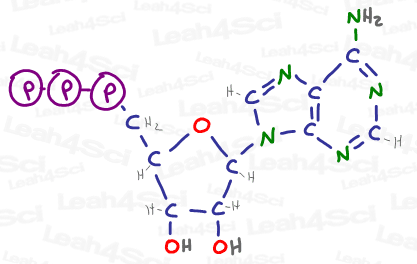
And if any of those other atoms are also carbon, we have the potential to build up a giant molecular structure such as ATP, drawn below, a source of energy and genetic building material within cells.
How does that work?
A review of carbon’s electron configuration shows us that carbon has a total of 6 electrons, with only 4 electrons in its valence shell.
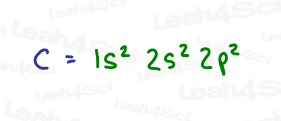
But, wait a minute!
Despite having 4 valence electrons,
There are not 4 empty spaces waiting to be filled… YET!
The 2s electrons in carbon are already paired and thus unwilling to accept new incoming electrons in a covalent bond.
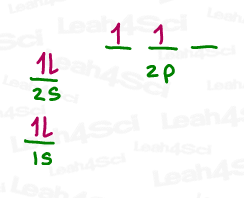
This leaves us with:
- 2 p orbitals, each with a single unpaired electron capable of forming ONE bond
- An empty p orbital, lacking the electron to initiate a bond
With its current configuration, carbon can only form 2 bonds,
Utilizing its TWO unpaired electrons,
Which isn’t very helpful if we’re trying to build complex macromolecules.
Enter Hybridization.
If we can find a way to move ONE of the paired s electrons into the empty p orbital, we’d get something like this.
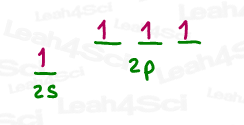
This can’t happen though, because the Aufbau Principle says that electrons must fill atomic orbitals from lowest to highest energy. This too is covered in my Electron Configuration videos.
So what do we do, if we can’t follow the Aufbau Principle?
Enter hybridization!
How does hybridization occur?
Here is how I like to think of hybridization.
We take that s orbital containing 2 electrons and give it a partial energy boost.
At the same time, we rob a bit of the p orbital energy.
This gives us 4 degenerate orbitals, meaning orbitals that have the same amount of energy.
Growing up, my sister and I shared a bedroom. We didn’t love it, but it made sense given that we’re both girls and close in age.
When we moved to an apartment with an extra bedroom, we each got our own space. Why would we choose to share once we had the option to have our own rooms?
Electrons are the same way. Now that we have a total of 4 degenerate orbitals and 4 electrons, why would we make them share a ‘room’ if they don’t have to?
Instead, each electron will go into its own orbital.

The number of electrons that move and orbitals that combine, depends on the type of hybridization we’re looking to create.
So let’s break it down.
Today, I will focus heavily on sp³, sp² and sp hybridization, but do understand that you can take it even further to create orbitals like sp³d and sp³d², as well (brief mention at the end).
Sp³ Hybridization
Watch the video below for a quick overview of sp³ hybridization with examples.
Let’s go back to our carbon example.
The most straightforward hybridization is accomplished by mixing the single 2s orbital containing 2 electrons, with all three p orbitals, also containing a total of 2 electrons.
Now that we have 4 degenerate unpaired electrons, each one is capable of accepting a new electron from another atom to create a total of 4 bonds.
But what do we call these new ‘mixed together’ orbitals?
They’re no longer s, and they’re no longer p.
Instead, they’re somewhere in the middle.
Being degenerate, each orbital has a small percentage of s and a larger percentage of p.
The mathematical way to describe this mixing is by multiplication.
By mixing 1s and 3p, we essentially multiplied s x p x p x p.
Think back to your basic math class.
If we have p times itself (3 times), that would be p x p x p
or p³.
And so EACH orbital is an s x p³ or sp³ hybrid orbital,
Because they were derived from 1 s and 3 p orbitals.
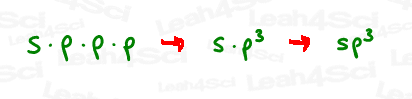
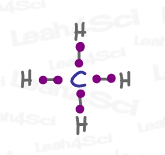
CH4 sp³ Hybrid Geometry
Let’s take the simple molecule methane, CH4.
Each sp³ orbital in carbon accepts an electron from a different hydrogen atom to form a total of 4 bonds.
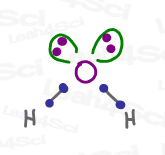
But this flat drawing only works as a simple Lewis Structure (video). Once you understand hybridization, you WILL be expected to predict the exact shape (Molecular vs Electronic Geometry, to be discussed shortly) as well as the bond angle for every attached atom.
So let’s dig a bit deeper.
Electrons are negative, and as you may recall,
Opposites attract (+ and -) and like charges repel.
And those negative electrons in the orbitals…
Well let’s just say they don’t like each other.
They repel each other so much that there’s an entire theory to describe their behavior.
The VSEPR Theory
VSEPR stands for Valence Shell Electron Pair Repulsion.
While electrons don’t like each other overall, they still like to have a ‘partner’.
And so they exist in pairs.
This could be a lone electron pair sitting on an atom, or a bonding electron pair.
For example, see water below. Oxygen has 2 lone pairs and 2 electron pairs that form the bonds between itself and hydrogen.
The one exception to this is the lone radical electron, which is why radicals are so very reactive.
The VSEPR theory, often pronounced ‘VES-per’ theory, tells us that an electron pair will push other electron pairs as far away from itself as possible.
If EVERY electron pair is pushing the others as far away as possible, they will find the greatest possible bond angle they can EACH take.
Sp³ Bond Angle and Geometry
While I ultimately want you to be able to draw and recognize 3-dimensional molecules without help, I strongly urge you to work with a model kit at first. Being able to see, touch and manipulate the shapes in real space will help you get a better grasp of these angles.
Watch this video to learn all about When and How to Use a Model Kit in Organic Chemistry.
When a central atom such as carbon has 4 equivalent groups attached (think: hydrogen in our methane example), VSEPR theory dictates that they can separate by a maximum of 109.5 degrees. That’s the sp³ bond angle.
The name for this 3-dimensional shape is a tetrahedron (noun), which tells us that a molecule like methane (CH4), or rather that central carbon within methane, is tetrahedral in shape.
You may use the terms ‘tetrahedron’ noun, or ‘tetrahedral’ adjective, interchangeably.
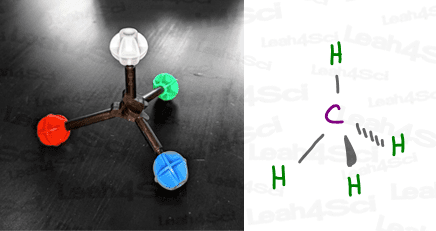
The carbon in methane is said to have a tetrahedral molecular geometry AND a tetrahedral electronic geometry.
Say what?
Molecular vs Electronic Geometry
When looking at the shape of a molecule, we can look at the shape adopted by the atoms or the shape adopted by the electrons.
Every electron pair within methane is bound to another atom.
But what if we have a molecule that has fewer bonds due to having lone electron pairs?
Molecular Geometry tells us the shape of the molecule itself, paying attention to just the atoms thus ignoring lone pairs.
Electronic Geometry tells us the shape of the electrons around the central atom, regardless of whether the electrons exist as a bond or lone pair.
Let’s take a closer look.
NH3 Hybridization and Geometry
Ammonia, or NH3, has a central nitrogen atom.
A quick review of its electron configuration shows us that nitrogen has 5 valence electrons.
N = [He] 2s² 2p³
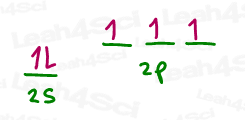
The next step is somewhat counterintuitive in that N appears to be able to form 3 bonds with its 3 p orbital electrons. However, lone electron pairs MUST BE the same energy as sigma bonds and so it STILL has to hybridize both its s and p orbitals.
These will be hybridized into four sp³ orbitals of which the first contains 2 (paired) electrons.
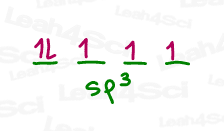
The remaining orbitals with unpaired electrons are free to each bind to a hydrogen atom.
While we expect ammonia to have a tetrahedral geometry due to its sp³ hybridization, here’s a model kit rendering of ammonia. Does it appear tetrahedral to you?
Not quite!
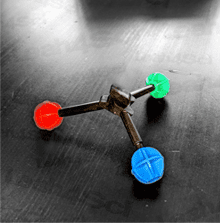
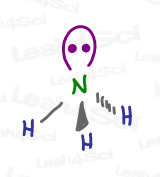
And yet, it IS still in fact tetrahedral, according to its Electronic Geometry.
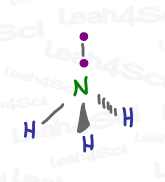 When looking at the electronic geometry, simply imagine the lone pair as an electron bound to its partner electron.
When looking at the electronic geometry, simply imagine the lone pair as an electron bound to its partner electron.
However, its Molecular Geometry, what you actually see with the kit, only shows N and 3 H in a pointy 3-legged shape called Trigonal Pyramidal.
Trigonal because it has 3 bound groups.
Pyramidal because it forms a pyramid-like structure.
Trigonal Pyramidal features a 3-legged pyramid shape.
H2O Hybridization and Geometry
This concept of molecular vs electronic geometry changes even more when the molecule in question, while still sp³, has 2 lone pairs and therefore only 2 bonds.
The water molecule features a central oxygen atom with 6 valence electrons.
O = [He] 2s² 2p4
Oxygen’s 6 valence electrons sit in hybridized sp³ orbitals, giving us 2 paired electrons and 2 free electrons.

Since water’s oxygen is sp³ hybridized, the electronic geometry still looks like carbon (for example, methane).
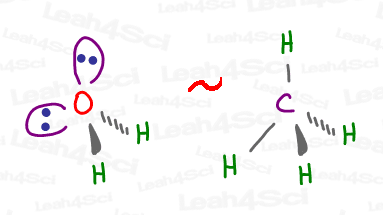
But the model kit shows just 2 H atoms attached, giving water the Bent Molecular Geometry.
Sp² Hybridization
What if I’m NOT looking for 4 degenerate orbitals?
What if I can get by with only 2 or 3 hybrid orbitals surrounding a central atom?
The video below has a quick overview of sp² and sp hybridization with examples.
Every bond we’ve seen so far was a sigma bond, or single bond. But you may recall that pi bonds are of higher energy AND that they utilize the p orbital, rather than a hybrid orbital.
While less common, empty orbitals (think carbocation) also exist with unhybridized p orbitals.
In order to create that pi bond or carbocation, we need to save a p orbital prior to hybridizing the rest. Take a look at the drawing below.
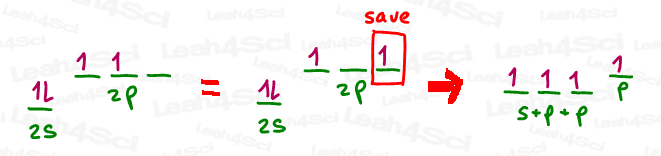
In the above drawing, I saved one of the p orbitals that had a lone electron to use in a pi bond.
Then, I mixed the remaining s orbital (two electrons) and 2 p orbitals (only one electron) to give me 3 brand new orbitals, containing a total of 3 electrons.
Since these orbitals were created with s and p and p, the mathematical result is s x p x p,
or s x p², which we can simply call sp².
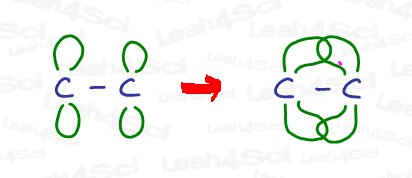
Reminder: A double bond consists of TWO bonds – a single or sigma bond, coupled with the second ‘double’ or pi bond. The sigma bond is no different from the bonds we’ve seen above for CH4, NH3 or even H2O. We simply add a pi bond on top of the sigma to create the double bond (and a second pi bond to create a triple bond).
The pi bond sits partially above and partially below the plane of the molecule as an overlap of the unhybridized p orbitals.
Sp² Bond Angle and Geometry
Let’s take a look at the central carbon in propanone, or acetone, a common polar aprotic solvent for later substitution reactions.
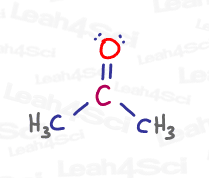
As you can see, the central carbon is double-bound to oxygen and single-bound to 2 methyl group carbon atoms. This gives carbon a total of 4 bonds: 3 sigma and 1 pi.
You’re most likely to see this drawn as a skeletal structure for a near-3D representation, as follows:

According to VSEPR theory, we want each of the 3 groups as far away from the others as possible. If you think of the central carbon as the center of a 360° circle, you get 360 / 3 = 120°
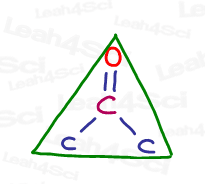
The technical name for this shape is trigonal planar.
Trigonal tells us there are 3 groups.
Planar tells us that it’s flat.
The sp² hybrid geometry is a flat triangle.
Since the carbon in acetone has no lone pairs, both its molecular geometry (what you see based on the atoms) and its electronic geometry (the configuration of electrons) are trigonal planar.
What if we DO have lone pairs?
As with sp³, these lone pairs also sit in hybrid orbitals, which makes the oxygen in acetone an sp² hybrid as well.
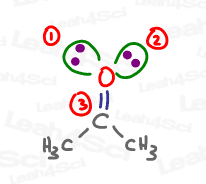
Ozone or O3 Hybridization and Geometry
Ozone is an interesting molecule in that you can draw multiple Lewis structures for it due to resonance. Let’s take a look at its major contributing structures.
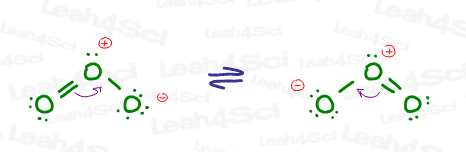
Ignoring the (+) and (-) formal charges, the central oxygen atom has one double bond (sigma and pi), one single bond (sigma only), and one lone pair. The 2 sigma bonds and 1 lone pair all exist in 3 degenerate sp2 hybrid orbitals.
While the trigonal planar Electronic Geometry is similar to acetone, when we look at JUST the atoms, we get a Bent shape for the Molecular Geometry.
BH3 or BF3 Hybridization and Geometry
Sp² hybridization doesn’t always have to involve a pi bond.
Take a molecule like BH3 or BF3, and you’ll notice that the central boron atom has a total of 3 bonds for 6 electrons. This is an allowable exception to the octet rule.
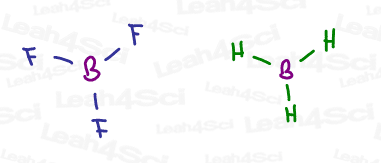
3 bonds require just THREE degenerate orbitals. By mixing s + p + p, we still have one leftover empty p orbital.
In the case of acetone, that p orbital was used to form a pi bond. In the case of boron, the empty p orbital just sits there empty, doing nothing, potentially waiting to get attacked, as you’ll later see in the Hydroboration of Alkenes Reaction.
I mean… who doesn’t want to crash an empty orbital?
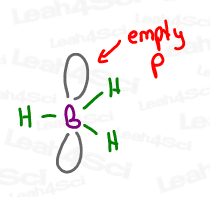
Sp Hybridization Bond Angle and Geometry
Review the video above (Start of the sp² section) for an overview of sp² AND sp hybridization.
An atom can have up to 2 pi bonds, sometimes with the same atom, such as the triple-bound carbon in HCN (below), or 2 double bonds with different atoms, such as the central carbon in CO2 (below).
In both examples, each pi bond is formed from a single electron in an unhybridized ‘saved’ p orbital as follows.
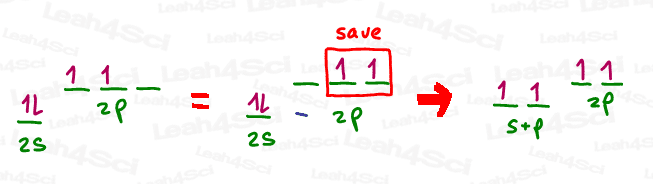
To achieve the sp hybrid, we simply mix the full s orbital with the one empty p orbital. The 2 electron-containing p orbitals are saved to form pi bonds.
Since this hybrid is achieved from s + p, the mathematical designation is s x p, or simply sp.
According to VSEPR theory, since the resulting molecule only has 2 bound groups, the groups will go as far away from each other as possible, meaning to opposite ends of the molecule.
This gives us a Linear shape for both the sp Electronic AND Molecular Geometry, with a bond angle of 180°.
HCN Hybridization and Geometry
We haven’t discussed it up to this point, but any time you have a bound hydrogen atom, its bond must exist in an s orbital because hydrogen doesn’t have p orbitals to utilize or hybridize.
The remaining C and N atoms in HCN are both triple-bound to each other.
 Notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.
Notice that, while carbon also has a single bond to hydrogen, the nitrogen has no other bond, just a lone pair.
Both of these atoms are sp hybridized.
Carbon has 1 sigma bond each to H and N.
N has one sigma bond to C, and the other sp hybrid orbital exists for the lone electron pair.
Both C and N have 2 p orbitals each, set aside for the triple bond (2 pi bonds on top of the sigma). This makes HCN a Linear molecule with a 180° bond angle around the central carbon atom.
CO2 Hybridization
Carbon dioxide, or CO2, is an interesting and sometimes tricky molecule because it IS sp hybridized, but not because of a triple bond.
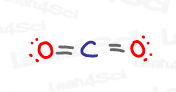
Take a look at the central atom.
Carbon is double-bound to 2 different oxygen atoms.
Each C to O interaction consists of one sigma and one pi bond.
The sigma bond requires a hybrid orbital, while the pi bond only requires a p orbital.
This means that carbon in CO2 requires 2 hybrid sp orbitals, one for each sigma to oxygen, and 2 untouched p orbitals, to form a single pi bond with both oxygen atoms.
THIS is why carbon is sp hybridized, despite lacking the expected triple bond we’ve seen above in the HCN example.
Interestingly, if you look at both oxygen atoms, you’ll notice that they each contain:
1 sigma bond
1 pi bond
2 lone pairs
Sigma bonds and lone pairs exist in hybrid orbitals. Since we need 3 hybrid orbitals, both oxygens in CO2 are sp² hybridized.
Hybridization Shortcut – Count Your Way Up
Back in general chemistry, I remember poring over a 2 page table, trying to memorize how to identify each type of hybridization. (We had to know sp, sp², sp³, sp³d and sp³d².)
You don’t have time for all that in organic chemistry.
Here’s how to determine Hybridization by Quickly Counting Groups:
1- Count the GROUPS around each atom in question.
The following each count as ONE group:
- Lone electron pair
- Single-bound atom
- Double-bound atom
- Triple-bound atom
In other words, groups include bound atoms (single, double or triple) and lone pairs.
2- Start reciting the orbitals in order until you reach that same number.
Using the examples we’ve already seen in this tutorial:
CH4 has 4 groups (4 H). Count 1, 2, 3, 4.
1: s
2: p¹
3: p²
4: p³
By simply counting your way up, you will stumble upon the correct hybridization – sp³.
NH3 has 4 groups – 3 bound H atoms and 1 lone pair.
1, 2, 3, 4 = s, p¹, p², p³ = sp³
The oxygen in acetone has 3 groups – 1 double-bound carbon and 2 lone pairs.
1, 2, 3 = s, p¹, p² = sp²
The central carbon in CO2 has 2 double-bound oxygen atoms and nothing else.
1, 2 = s, p¹ = sp
Right-Click the Hybridization Shortcut Table below to download/save.
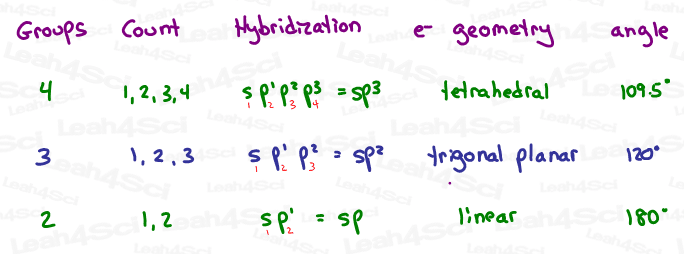
Sp³d and sp³d² Hybridization
While sp³d and sp³d² hybridization are typically not covered in organic chemistry, and less commonly discussed overall, you still see them on your MCAT, GAMSAT, PCAT, DAT or similar exam.
Follow the same trick above to see that sp³d hybridization occurs from the mixing of 5 orbitals (1s, 3p and 1d) to achieve 5 ‘groups’, as seen in the Phosphorus pentachloride (PCl5) example below.
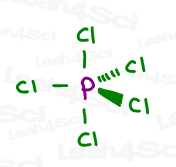
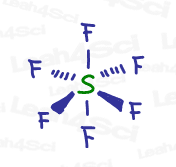
Sp³d² hybridization occurs from the mixing of 6 orbitals (1s, 3p and 2d) to achieve 6 ‘groups’, as seen in the Sulfur hexafluoride (SF6) example below.
In Conclusion
Hybrid orbitals are created by the mixing of s and p orbitals to help us create degenerate (equal energy) bonds. sp³ , made from s + 3p gives us 4 hybrid orbitals for tetrahedral geometry and 109.5 degree bond angles. Sp², made from s + 2p gives us 3 hybrid orbitals for trigonal planar geometry and 120 degree bond angles. sp made from 1 each s and p gives us a linear geometry with a 180 degree bond angle.
Ready to apply what you know? Try the practice video below:




