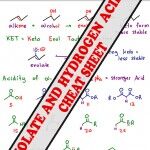
Alpha Hydrogen acidity comes from the ability to resonate negative conjugate electrons onto the nearby carbonyl group. Your professor may ask you to memorize pKa values. I recommend UNDERSTANDING this chart as explained in the enolate video so that you’re not left fumbling for numbers. <– Back to the Enolate Reactions Video Tutorial Series Homepage
Enolate Ion Formation for Alpha Carbonyl Reactions
Reactions at the alpha carbonyl position with enolate intermediates occur in many reactions! This includes but is not limited to: Alpha-Halogenation, Haloform & Idoform tests, Aldol, Claisen Condensation and more. Once you grasp enolate anion formation, it’s reactivity and stability, you’ll have the clarity you need to master the upcoming reactions. This video also covers common enolate pKa […]
Alpha Halogenation Reaction of Ketones and Aldehydes
Carbonyl groups provide a great starting point for synthesis reactions due to the reactivity at the alpha carbon position. Alpha halogenation is a great reaction to introduce leaving groups at the alpha position for further substitution or elimination reactions. Alpha halogenation can take place under acidic or basic conditions. The video below walks you through […]
Haloform Reaction and Iodoform Test
The Haloform Reaction is a very specific type of alpha halogenation reaction. This reaction takes place at the methyl ketone turning the methyl into a good haloform leaving group. This can include chloroform and bromoform, and of course the Iodoform solid precipitate used in lab to test for the presence of a methyl ketone. The […]
Aldol Addition and Condensation Reaction Mechanism
Aldol reactions are useful in organic chemistry synthesis for creating new carbon to carbon bonds. This reaction can occur as an aldol addition reaction at lower temperatures forming a beta-hydroxy aldehyde or ketone, or as an aldol condensation reaction at higher temperatures forming an alpha-beta unsaturated product. This video walks you through the addition and […]