Electrochemistry, as the name implies, is a topic that deals with the relationship between electrical potential and chemical reactions. Why is that relationship important, you ask? We can use electrical current to drive a nonspontaneous chemical reaction, or we can use a spontaneous reaction to drive an electric current (and store it!). And since electrochemistry […]
Intro to Balancing Redox Reactions
Balancing Redox Reactions is about more than just balancing atoms. Since oxidation and reduction involve the transfer of electrons, we also have to keep an eye on atoms and charge. Of course, if you’re wondering how redox reactions apply to the Galvanic/Voltaic or Electrolytic cell, visit my Electrochemistry series as well. Balancing Redox Equations Video […]
Oxidation and Reduction Reactions
Oxidation and Reduction reactions happen all the time in a living system and thus are very likely to show up on the MCAT. This video walks you through oxidation and reduction reactions, how to figure out the half reactions, and how to easily recognize (+ trick) what gets oxidized and what gets reduced. You’ll also […]
Calculating Oxidation Numbers in MCAT General Chemistry
Oxidation numbers can be confusing, unless you think of it as charge (real or imaginary) associated with each specific atom in a complex molecule or polyatomic ion. Calculating Oxidation Numbers Video 1 – Oxidation Numbers This video shows you how to find and calculate oxidation numbers when given simple and complex molecules or ions. (Watch […]
Common Types of Chemical Reactions
While the MCAT will not test you directly on the different types of chemical reactions, you will be expected to know them as they show up in more complex questions, passages, and experiments. This video walks you through the common types of chemical reactions including Disproportionation, Single Replacement, Double Replacement, Combination or Synthesis, Decomposition, and […]
Balancing Chemical Equations with Practice Problems
When it comes to balancing chemical equations, it’s very easy to get lost and confused by all the changing numbers. The videos below will show you a simple yet efficient approach to make sure you quickly account for all your numbers (minimal guessing) and don’t lose track of your work along the way. Balancing Equations […]
Mole and Avogadro’s Number in MCAT General Chemistry
Moles and Avogadro’s number are big! And they come across as overwhelming. But YOU can master them! This video covers the definition of Moles, with an illustration showing the logic of moles in general chemistry. You’ll also see how to easily and quickly convert from and to them on the MCAT. You’ll see conversions including […]
Conventions for Writing Chemical Equations – Arrows, Phases, Coefficients and more
When it comes to writing chemical equations it’s easy to confuse all the little letters and numbers. What do they mean? Where do they go? This video breaks down every letter and number you’ll see in chemical reactions, from coefficients and phases to numbers that represent charges, multiple atoms or polyatomic ions. (Watch on YouTube: Chemical […]
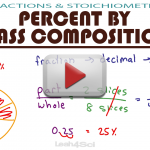
Percent By Mass Composition MCAT General Chemistry
All the composition by percent mass questions don’t have to be confusing. You can use them to get the information you need! Learn ONE rule for them all! This video makes this concept simple. You’ll see WHY and HOW these equations work, instead of memorizing all the different percent formulas. You will see examples worked step-by-step my […]
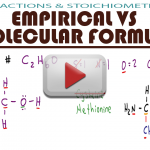
Empirical Formula and Molecular Formula Calculations
Navigating through empirical formulas versus molecular formulas will help you in conversions, unknowns, and so many exam problems. This video explains the definitions, similarities, and differences between an atom’s empirical formula and its molecular formula. You will also see when the empirical and molecular formulas are the same, and a percent by mass example! (Watch […]