Below is the written transcript of my YouTube tutorial video Acids and Bases in Organic Chemistry – Introduction to Acids and Bases in Organic Chemistry Video
Below is the written transcript of my YouTube tutorial video Acids and Bases in Organic Chemistry – Introduction to Acids and Bases in Organic Chemistry Video
If you prefer to watch it, see the video HERE, or catch the entire Acids and Bases series HERE
(click here to see the video on YouTube)
[Start Transcript]
Leah here from leah4sci.com and in this video series I’ll teach you what you need to know about comparing acid and bases in Organic Chemistry starting from understanding how Pka and Ka values relate to acid base strength without doing calculations followed by the factors you want to look at in comparing stronger to weaker acids and finally we’ll do some practice problems to determine which direction in the equilibrium is favored in the reaction.
You can find my entire series on Acids and Bases along with the Acid Base Cheat Sheets and Acid Base Practice Quiz by visiting my website leah4sci.com/AcidBase.
Recall from General Chemistry when studying acid and bases, you went through ka and pKa values, Kb, pKb, iso charts and lots and lots of tedious math. In organic chemistry you’re likely not looking at any Math in Acids and Bases instead you’re going to learn how to compare molecules by understanding them by analyzing different components of the reaction to determine which one is stronger or weaker and which side the reaction will favor. No numbers, no charts, just pure logic.
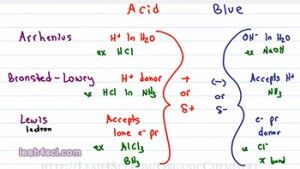
So the first question you have to ask yourself is What is an Acid and what is a Base? In General Chemistry you learn three different definitions for the Acid and Bases and these will still come up so let’s start with that. The first is the Arrhenius definition which tells you that an acid is anything that donates an H+ in solution specifically in an Aqueous or water solution and a Base is anything that will give you an OH minus (OH-) in an Aqueous solution. Examples include HCL when I drop HCl in water it breaks up to give me an H plus and CL minus and for a base, NaOH, it dissolves to give me an Na plus and OH minus.
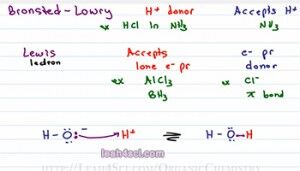
The next definition is Bronsted-Lowry and don’t think of this as a new definition, think of this as a more complete definition because a Bronsted-Lowry is something that will donate an H plus in solution, they will call it an H plus donor and that solution does not have to be water. For example, if I take HCL and I dissolve it in ammonium which is NH3, it will still break up to give me H plus and Cl minus. But because it’s not water, it’s not an Arrhenius acid but the fact that it still donates an H plus makes it a Bronsted-Lowry acid. Now since we are not dealing with water, our base is not necessarily OH minus but simply any ion or molecule that can accept an H plus in solution. So the bronsted-lowry base is something that accepts a proton or an H plus.
You’ll hear me use proton or H plus interchangeably, I wanna make sure you understand that they’re the same exact thing. A Hydrogen atom is something that has one proton and one electron. if we have deuterium, the isotope then it has one proton, one neutron, and one electron. If I take that Hydrogen atom and turn it into Cation or positive ion, what I’m doing is removing that electron, removing that negative which balances the positive and that leaves me with an H plus which is simply a proton because there’s nothing else there. So an H plus ion is a proton, don’t forget that. An example of a Bronsted-Lowry base, if we’re looking at the Ammonia solution would be Ammonia because the ammonia will react with the Hydrochloric acid and accept that proton to give you NH4 plus.
The final definition is the Lewis Acid and Base and this is different than the other two because we’re not looking at the transfer of a proton between two molecules instead we’re looking at electrons. And the way I remember this is I think of Lewis having to do with electrons. A Lewis acid is something that accepts a lone pair of electron, e minus is how I’m going to refer to electrons and pr for pair and a Lewis base is something that donates a lone pair of electron, we’ll call it an electron pair donor.
A good example you’ll see for a lewis acid is AlCl3 or even BH3 which show up in your reaction and the example for base could be anything with a lone pair of electrons for example Cl minus or even a pi bond as you’ll see with your alkene addition reactions. So most students memorize the three definitions and then they’re face with a reaction and they don’t know where to go.
Keep in mind these definitions are not mutually exclusive; you can have a molecule fall into more than one category. The purpose of these three categories is simply how to look at the acids and bases regardless of water, another solution or even no proton being transferred.
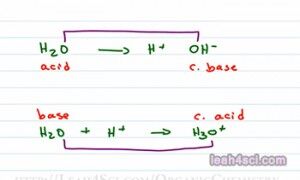
And I’m gonna prove that to you with an example. We’ll show what happens with an OH minus reacts with H plus to give me a product in equilibrium that is H2O. You should recognize this as the reverse reaction of the deionization of water. The reaction takes place as follows: OH minus will use one of its lone pair of electrons to reach out for and grab the red hydrogen. The electrons that reach for hydrogen now form a bond and we have a molecule of water. Since water is the product, let’s assume that this is taking place in water making an aqueous reaction and we can also assume that OH minus came from NaOH and H plus came from HCl. According to the Arrhenius definition the acid gives me an H plus in water which we have. The base gives me an OH minus in water which we have so this classify as Arrhenius acid and base.
The Bronsted-Lowry definition tells me that I have an H plus donor and H plus acceptor, well guess what? When OH minus attacks H plus it’s accepting the proton and the Cl minus that gave up that H plus donated a proton so it falls under the Bronsted-Lowry category as well.
And lastly the Lewis definition has to do with electrons. Well how does the OH minus reach out for and grab that H plus? It uses a lone pair of electrons which means that OH minus is a Lewis base donating its electrons and H plus is a Lewis acid because it’s accepting electrons. Most likely you’re going to look at the Arrhenius and Bronsted-Lowry more than Lewis in this topic of determining strengths but no one can fall under more than one category.
So now we know the definitions and we also know that they can be the same or different which makes it even more confusing. What am I looking for in an acid or a base? This is where you really need to understand rather than go through this checklist in your mind everytime because the questions are gonna take too long to solve this way.
So let’s take a look. If I have H plus coming off a molecule, that molecule is likely positive or partially positive because otherwise it would not give up an H plus. If I have the Arrhenius acid giving up an H plus and a Bronsted-Lowry giving up an H plus, the molecule, in order to able to give up that H plus, must be positive or partially positive. It must be positive or partially positive. HCl has a partially positive Hydrogen. H2SO4 partially positive Hydrogen, Alcohol, partially positive Hydrogen and then we look at the Lewis acid. If something is going to accept the lone pair of electrons, those electrons are negative or electronegative. And if opposites attract and I’m not gonna have a negative molecule accepting a lone pair of electrons. In other words, the Lewis acid must be positive or partially positive in order to accept a lone pair of electrons. So what you’ll notice here is that the acid regardless of the definition is going to be positive or partially positive.
Now let’s look at the bases. The Arrhenius base is OH minus, OH minus is negative. The Bronsted-Lowry base accepts a proton. It’s accepting something positive which means it could be negative like OH minus or partially negative like the lone pair of electrons in ammonia acting as base. And finally our Lewis base is donating a pair of electrons. In order to have that pair of electrons to give away, the atom or molecule must be negative or electronegative; otherwise it will not give away its electrons. So the trend for the bases is simply something that is negative or partially negative.
So now you are looking at two molecules and comparing them in a reaction and trying to figure out which is the acid or the base. Unless you’re specifically asked for the Arrhenius, Bronsted-Lowry or Lewis acid or base, just look for the one that is more positive as your acid and the one that is more negative as your base especially when comparing a molecule and its own conjugate, because an acid will become more negative as a conjugate base and a base will become more positive as a conjugate acid.
So we understand the definitions, now we have to know how to determine what is an acid and what is a base. So if I give you a molecule H2O, is that an acid or is that a base? Well let’s see. An acid would break up to give me H plus, a base would be me OH minus. What if it can do both? So what you need to do is look at your molecule in context of the reaction or in comparison to another atom and see what you can come up with.
I can show you the water acting as both an acid and a base depending on which reaction I’m looking at. If water breaks up to give me an H plus and then OH minus, then water is giving up a proton making it an acid. On the other hand if water is going to react with and accept a proton to give me H3O plus then water acts as a base because it’s accepting a proton. Now this is the proper way to look at it but in reactions you don’t necessarily see the individual ions, you may see molecule A and B forming molecule C and D. So here’s another way that I like to look at it. Compare the molecule that reacted. In this case we have an oxygen and an oxygen so these two are conjugates. When an acid reacts it forms a conjugate base because the molecule it forms is a base. When a base reacts, it forms a conjugate acid. Conjugate simply means the thing that formed at the other side of the reaction. And here we’ll compare again the species containing Oxygen. Here’s what you’re going to look at when determining if it’s an Acid or a Base.
So as we said before, we’re looking for the one that is more positive as the acid more negative as the base. H2O versus OH minus, we’re comparing neutral to negative. The neutral is more positive thatn the negative making it an Acid and OH minus is more negative and something neutral making it the conjugate base. For the second reaction H2O being neutral is more negative than a positive H3O plus making this the base and H3O plus is more positive than the neutral water making it the conjugate acid. Now what if you’re not given a reaction, what if you simply have two molecules and you see Ka or pKa values or what if you’re not given any values and you’re asked to compare them. That’s exactly what will discuss in the next video which you can find on my website alongwith the acid-base cheat sheet and acid-base practice quiz leah4sci.com/AcidBase.
Are you struggling with Organic Chemistry? Are you looking for resources and information to guide you through the course and help you succeed? If so, then I have a deal for you, a FREE copy of my eBook “10 Secrets to Acing Organic Chemistry”. Use the link below or visit orgosecrets.com to grab your free copy. After downloading your free copy of my eBook, you’ll begin receiving my exclusive email updates with Cheat Sheets, reaction guides, study tips and so much more. You’ll also be the first to know when I have a new video or live review coming up.
If you enjoyed this video, please click the thumbs up and share it with your Organic Chemistry friends and classmates. I will be uploading many videos over the course of the semester so if you haven’t subscribed to my channel yet, do so right now to be sure that you don’t miss out.
[End Transcript]
Click here to Catch my entire video series on Acids and Bases or click HERE for the ACID/BASE practice quiz