 Below is the written transcript of my YouTube tutorial video EAS Video #1 – Introduction to Electrophilic Aromatic Substitution
Below is the written transcript of my YouTube tutorial video EAS Video #1 – Introduction to Electrophilic Aromatic Substitution
If you prefer to watch it, see the video HERE, or catch the entire EAS video series HERE
(click here to see the video on YouTube)
[Start Transcript]
Leah here from leah4sci.com where you can find hundreds of organic chemistry tutorial videos, orgo cheat sheets, study tips and more. And in this video I will introduce the topic of EAS for electrophilic aromatic substitution. The bad news is many students find this topic difficult, but the good news is this is actually an easy and fun topic if you understand what’s going on and my goal with this video series is to make sure that you know exactly how to proceed with reactions.
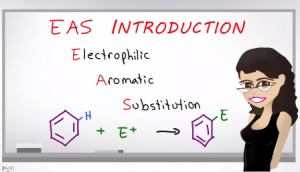
But first we have to break down EAS to understand what’s going on. Electrophilic comes from the word electro meaning electron, Philic or phile meaning loving. An electrophile is something that is electron loving. Since opposite attract, something that is electron loving must be positive or partially positive and so we know this reaction is going to take place using molecules that are positive or partially positive. Aromatic comes from aromaticity which means a molecule that is cyclic plainer conjugated and obeys Huckle’s rule.
Most of these reactions will take place with benzene but you’ll also see some other related compounds. And finally we have substitution, which means we replace one group with another. In this case, we’re going to replace the hydrogen with a group that’s adding to the ring.
Let’s have a quick overview :
The EAS reaction typically starts out when a benzene reacts with a super electrophile which we’ll explain shortly. The final product that has the electrophile attached to the benzene ring. This appears to be addition but it’s really a substitution reaction, because don’t forget, we used to have a hydrogen here, and when that electrophile attaches to benzene, the hydrogen has to be removed and this is something we’ll look at in the actual mechanism.
I mentioned that this reaction requires a super electrophile and let’s see why:
By reviewing an alkene addition reaction. Say I have 2-butene reacting with bromine. The nucleophilic pi electron will reach out for bromine, bromine attacks back and the second bromine breaks off. I have a bridge intermediate and so the negative bromine comes and attacks from the opposite side breaking the bridge and giving me the final product an anti addition vicinal dihalide. If you’re not comfortable with this reaction, go back to my alkene reaction video series where I go through it in detail.
But what I want to show you here is that pi electrons are very nucleophilic and will attack when given the opportunity. And so it’s only natural to think that if we can add something to an alkene we should be able to carry out the same exact reaction with Benzene since benzene has three Pi bonds. In fact, shouldn’t we have triple the product? But in reality, if you try adding bromine to benzene using the same reaction conditions you are going to get absolutely no reaction.
Why? Because the Pi bonds in Benzene are a lot more stable than an alkene. In reactions, we follow the rules of happy stables unreactive, unhappy, unstable, very reactive. The only reason a molecule will react is of the next step where the outcome will make it more stable than the starting molecule. But with benzene, if you were to break open the ring for an addition reaction, you’re sacrificing the aromaticity or sacrificing the stability and it’s not worth it. Since benzene will not reach out to attack a standard electrophile, we need something that is so positive and so unstable that benzene has no choice to attack and this is the idea of the super electrophile.
If you compare your EAS reactions to your alkene reactions, you’ll notice that the super electrophiles are so much more positive and so much less stable as compared to your standard alkene electrophiles. Now that you have the idea of what goes on in EAS and what to expect, join me for the remainder of this series where I take you through the mechanism for electrophilic aromatic substitution as well as individual videos devoted to the formation of the super electrophile for the most common reactions that you’ll cover including:
Halogenation, Nitration, Sulfonation Fridel crafts alkylation and friedel crafts acylation. Once you have those basics in place, we’ll continue with directing effects and new multiple substitutions to make sure that you really get this concept. You can find my entire video series on Electrophilic Aromatic Substitution by visiting my website leah4sci.com/EAS.
Are you struggling with Organic Chemistry ? Are you looking for resources and information to guide you through the course and help you succeed? If so, then I have a deal for you! A free copy of my ebook – 10 Secrets to Acing Organic Chemistry. Use the link below or visit orgosecrets.com to grab your own copy. After downloading your free copy of my ebook, you’ll begin receiving my exclusive email updates with cheat sheets, reaction guides, study tips and so much more. You’ll also be the first to know when I have a new video or live review coming up.
If you enjoyed this video, please click the thumbs up and share it with you organic chemistry friends and classmates. I will be many videos over the course of the semester so if you haven’t subscribed to my channel yet, do so right now to be sure that you don’t miss out! [End Transcript]
Click here to Catch my entire video series on Electrophilic Aromatic Substitution


