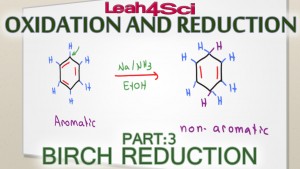
 Aromatic compounds are considered very stable thus difficult to react.
Aromatic compounds are considered very stable thus difficult to react.
Birch Reduction is one example of an extreme reaction strong enough to break benzene's aromaticity to form an non conjugated cyclohexadiene.
This video breaks down the reaction and mechanism, followed by my trick for quickly identifying the product when EDG/EWG substituents are present.
(Watch on YouTube: Birch Reduction. Click cc on the bottom right for video transcript)
<–Watch Previous Video: Oxidation & Reduction Alkenes and Alkynes
–>Watch Next Video: Alcohol Oxidation to Aldehyde, Ketone, and Carboxylic Acid
This is video 3 in the Orgo Oxidation/Reduction Series. Click for complete series
Ready to test your redox skills? Try the Redox Practice Quiz and follow along with the Redox Cheat Sheet



How come the first pi bond shifted to make another but on a different carbon whereas the second pi bond collapsed as a lone pair?
u are great great great from ur fan
Thanks, Ishan!
If two group of EWD and i molecule of alkene is attached with benzene ring than what happens.