Understanding and drawing Fischer projections is a critical skill for many organic chemistry students and definitely a critical skill for biochemistry and your MCAT/DAT or similar exam.
Understanding and drawing Fischer projections is a critical skill for many organic chemistry students and definitely a critical skill for biochemistry and your MCAT/DAT or similar exam.
This tutorial series will take you through the very basics of Fischer Projections, all the way through the advanced biochemistry concepts. Download my free Fischer Projections Cheat Sheet to follow along with the tutorials.
Once you've made it through the series, test your knowledge by working through my Fischer Projections Practice Quiz with solutions.
Do you need a Model Kit? Watch How to Use Your Organic Chemistry Model Kit.
Or, are you struggling with drawing Fischer Projections and feeling like you need some real art skills to master them? Read Organic Chemistry or Art Class?
Let's start now, at the very beginning:
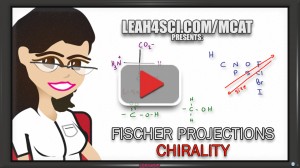
What are Fischer Projections? What do they represent?
Video 1 – Introduction to Fischer Projections
Once you understand what they are, you have to be able to classify their chirality centers. That's exactly what's covered in video 2 – from simple single-carbon structures to more complex multi-chiral center molecules.
Video 2 – Fischer Projection Stereochemistry
Converting Fischer Projections
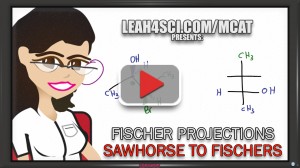
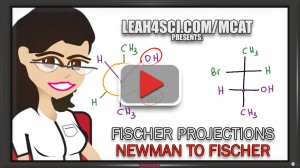
 But Fischer projections cannot be studied in vacuum. To truly understand them you must be comfortable converting to/from Sawhorse projections, Newman projections, even Haworth and Chair structures for sugar molecules.
But Fischer projections cannot be studied in vacuum. To truly understand them you must be comfortable converting to/from Sawhorse projections, Newman projections, even Haworth and Chair structures for sugar molecules.
If you have trouble visualizing these 3-D conversions, I recommend picking up a simple model kit. Here's the one I use and recommend. (If you purchase through this Amazon link I receive a small commission at no extra cost to you.)
Video 3: Sawhorse to Fischer and back
Video 5: Carbohydrate Fischer to Haworth Shortcut
Need help drawing 6-membered rings and chairs? See my tutorial on drawing chairs and ring flips.
Feel confident with Fischer Projections? Click to try the Practice Quiz