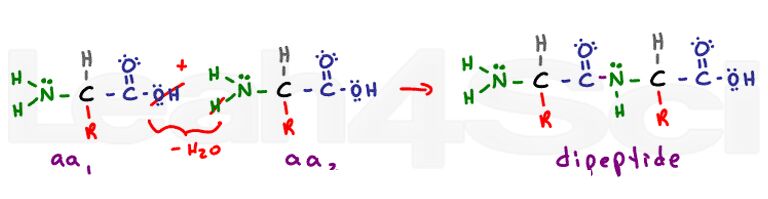
Amino acids are the building blocks of living things. Long chains of amino acids make up proteins, which in turn make up many structural and functional cell components.
Amino acids are the building blocks of living things. Long chains of amino acids make up proteins, which in turn make up many structural and functional cell components.
I like to think of the cell as a self contained city where the nucleus is the capital, the mitochondria is the power plant and so on. But then you have your workers, transportation system and the very structure of the cell city, which are all made of proteins- which in turn are made of amino acids.
The complexity of a protein structure is determined by its sequence of amino acids and the chemical nature of their variable group side chains. The MCAT requires understanding the nature of polar and nonpolar side chains and the twisting and conformations caused by hydrophobic and hydrophilic interactions
And yes, you should be memorizing each amino acid for the MCAT. This includes the side chain, full name, 3-letter name and single letter abbreviation. But don’t simply stick the words and structures onto flashcards hoping to force it into your memory. You need to actively tackle each amino acid individually.
For a quick reference list download my free Amino Acid Cheat Sheet Study Guide
- Write out the full name
- Draw the amino acid structure and variable group
- Write out the 3-letter and single letter abbreviations
- Verbalize something unique about this specific side chain- out loud! The more funny and crazy the connection, the easier you will remember it.
- Repeat the naming/drawing process once RIGHT AWAY
- Repeat weekly until you feel SOLID with this information
The Basic Structure of an Amino Acid
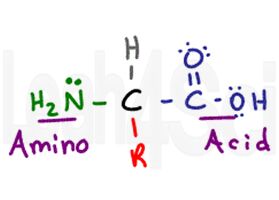 The amino acid gets its name from its two primary functional groups. The amino acid has a central chiral carbon called the alpha carbon (black). Attached to the central carbon you have a hydrogen atom (gray), an amino or NH2 group (green), and a carboxylic acid COOH group (purple). Finally we have the R group (red), which is a variable side chain.
The amino acid gets its name from its two primary functional groups. The amino acid has a central chiral carbon called the alpha carbon (black). Attached to the central carbon you have a hydrogen atom (gray), an amino or NH2 group (green), and a carboxylic acid COOH group (purple). Finally we have the R group (red), which is a variable side chain.
There are 20 different amino acids distinguished by their unique side chains. They range from a simple hydrogen atom (glycine) to a complex 2-ring resonating aromatic system (tryptophan).
While the fully-neutral version above is how most students study amino acids, and the form we’ll use in this article, keep in mind that this is technically incorrect.
Amino Acid in Zwitterion Form
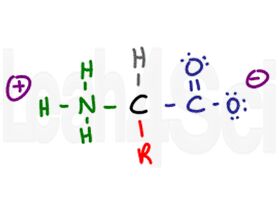
Since the carboxyl group is acidic and the amino group basic, the two will exist as a zwitterion in their conjugated charged forms in physiological pH. More on zwitterion and amino acid charges in my next article (link to follow).
One final concept before we break down the individual amino acids, and that is the 3-dimensional protein structure. In a biological system structure determines function, so understanding amino acid characteristics is key to understanding structure and ultimately protein function.
Primary Structure of a 3-D Protein
The first and more important determination factor of protein structure is the sequence of amino acids. If the polypeptide chain is attached in a different order, you get a very different overall structure.
Secondary Structure of a 3-D Protein
The secondary structure comes from backbone hydrogen bonding interactions. The peptide bond turns every former carboxyl and amino group into an amide functional group. The secondary structure of alpha helix and beta pleated sheets come from hydrogen bonding between the partially negative oxygen on the carbonyl and the partially positive hydrogen on the nitrogen.
Tertiary Structure of a 3-D Protein
The tertiary structure is where the real 3-dimensional folding is introduced, and this is the first time you’ll notice side-chain interactions. THIS is where knowledge and understanding of amino acid side chains are critical.
Let me repeat myself, tertiary structure is the first time you’ll see variable R-group side chain interactions on the polypeptide chain. Many students confuse this with secondary structure, which is only backbone interactions.
Quaternary Structure of a 3-D Multi-Polypeptide Protein
Quaternary structure refers to the variable group interactions between different polypeptides to form a single larger protein.
Quaternary structures are not found in every protein. If the protein contains just a single amino acid strand, then the highest level of folding is its tertiary structure. However, if the protein is made up of multiple polypeptide subunits, then the quaternary structure is what holds the different polypeptides together.
Now that you understand the significance of side-chain characteristics, let’s dive into amino acids. Keep in mind that since the parent amino and carboxyl groups are ‘busy’ with primary/secondary structure, they are NOT analyzed when studying side-chain properties and characteristics.
This means you ignore any potential polarity on both the carboxyl and amino groups and ONLY look at the side chains.
Non-Polar Hydrophobic Amino Acids
Hydrophobic, as the name implies is hydro – water, phobic – fearing. Hydrophobic amino acids have little or no polarity in their side chains. The lack of polarity means they have no way to interact with highly polar water molecules, making them water fearing.
There are only five atoms that will appear in your amino acid variable groups: H, C, N, O, and S.
Only consider polarity when you have N, O, S as the ‘majority’ factor. I’ll point these out as they occur. However, if all you see are Cs and Hs you should automatically recognize a water-fearing amino acid.
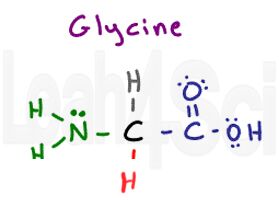
Glycine
Gly
G
Glycine is a unique amino acid in that it doesn’t really have an R group. When you think ‘variable R group,’ you should think of carbon attached to other atoms. But glycine only has a hydrogen at its side chain position. Since glycine has 2 hydrogen atoms, one each on the parent and side chain, it’s the only symmetrical and thus achiral amino acid. Since hydrogen is non-polar, glycine is a hydrophobic amino acid. The Hydrogen side-chain makes glycine the smallest amino acid.
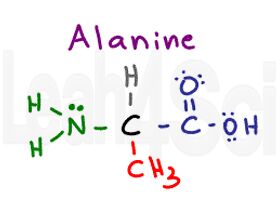
Alanine
Ala
A
Alanine is a simple amino acid which has just a methyl or CH3 group as its side chain. Since you see nothing but carbon and hydrogen, Alanine is a non-polar hydrophobic amino acid. It’s important to recognize that this is a very small amino acid and capable of being ‘wedged’ into tight loops or chains.
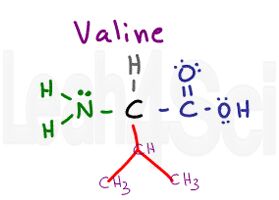
Valine
Val
V
Valine is another simple amino acid with just an isopropyl variable group. Just like alanine, we see nothing but carbon and hydrogen, making valine a non-polar hydrophobic amino acid.
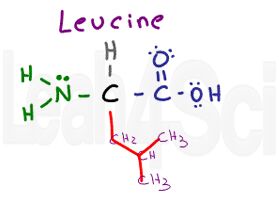
Leucine
Leu
L
You can recognize leucine as having the same variable group as valine but with an extra CH2 group. Or you can simply recognize its isobutyl side chain.
See this video if you’re not familiar with branched side chains like isopropyl or sec-butyl. Since leucine has just Cs and Hs, it’s a water fearing non-polar amino acid.
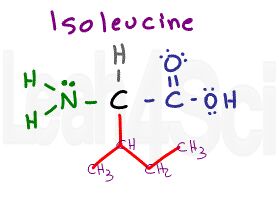
Isoleucine
Ile
I
Isoleucine, as the name implies, is an isomer of leucine. The difference is the placement of the CH3 for a sec-butyl rather than a isobutyl side chain. Just like its isomer, isoleucine is nonpolar and hydrophobic.
Methionine
Met
M
Methionine is the first potentially tricky amino acid. It’s side chain contains mostly Cs and Hs but with an embedded sulfur atom. While you may think it’s hydrophilic, pay careful attention to the location of the sulfur atom. Embedded in the chain and attached to just carbon atoms, sulfur is partially ‘hidden’ from the outside environment.
While you don’t have to know electronegativity values for the MCAT it helps to understand that S = 2.58 and C = 2.55. Since the difference in electronegativity is less than 0.5, there is NO polarity on this sidechain.
You must also recognize methionine as the start codon AUG in RNA translation to proteins.
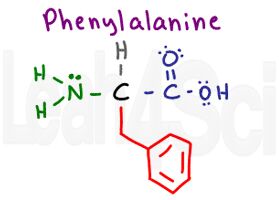
Phenylalanine
Phe
F
To help you remember that Phenylalanine is F remember that ‘ph’ is pronounced as an ‘F’. Don’t mix this up with P for proline.
Pay attention to the structure of phenylalanine. It has a single carbon group with an attached benzene ring. Phenyl is the name for a benzene substituent, and this molecule has a benzene (phenyl) attached to the structure of alanine. Since phenylalanine has nothing but Cs and Hs in its aromatic side chain, it is nonpolar and hydrophobic.
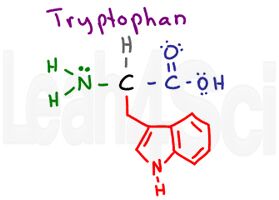 Tryptophan
Tryptophan
Trp
W
This is a tricky one. Notice the N-H in this side chain. N-H should be polar and capable of hydrogen bonding. However, there are two reasons this amino acid is still non-polar and hydrophobic.
- The N-H group is a tiny portion of the very large side chain.
- Look carefully at nitrogen, and more importantly, at its lone pairs. Nitrogen’s electrons are integral to the conjugated aromaticity for the tryptophan side chain. In other words, think of its electrons as ‘too distracted’ by resonance to pay much attention to the external water environment.
The MCAT requires you to recognize that this is a large and bulky amino acid. But since it’s a multiple choice exam you can simply memorize that tryptophan is the ONLY amino acid with TWO fused rings.
In fact, it’s so big it can TRIP (trp) over itself and cry out Waaaaaa (W)
(Note on mnemonics: The funnier, weirder, or dirtier the mnemonic, the more likely you will remember it. Keep this in mind for med-school.)
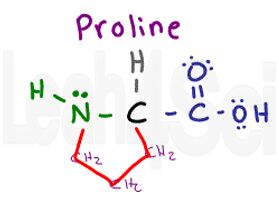 Proline
Proline
Pro
P
Proline is a unique amino acid since it’s THE ONLY ONE that incorporates the backbone into its side chain. The proline side chain is a 3-carbon chain that loops around and attaches back to the parent amino group. This means that unlike the other amino acids, proline does NOT have a hydrogen atom on its nitrogen when part of a polypeptide chain.
However, you cannot forget that nitrogen is NOT REALLY part of the variable group, which means it cannot contribute any polarity. Since we just have 3 CH2 groups to analyze we get a nonpolar hydrophobic side chain. This parent-loop creates a bulge and does not allow a proline-containing chain to be linear, which means you’ll often find it in loops and at the end of an alpha helix.
Polar Hydrophobic Amino Acids
*This is a sticky section, and depending on where you research you may find the following categorized as polar or nonpolar, hydrophilic or hydrophobic. Pay attention to the presence of polar groups that are small compared to the overall sidechain, or very weakly polar and therefore hydrophobic.
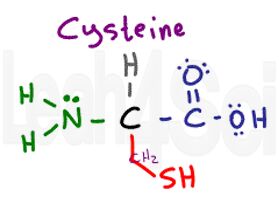 Cysteine
Cysteine
Cys
C
Cysteine has a slightly polar S-H, but its polarity is so mild that cysteine is unable to properly interact with water making it hydrophobic.
Cysteine is a very important amino acid when it comes to tertiary and quaternary structure. Most side-chain interactions include polar/charged interactions or non-polar Van Der Waals and London dispersion. However, cysteine’s side chain is capable of forming a disulfide bridge, which is a covalent bonds between 2 sulfur atoms through side chain oxidation and removal of 2 hydrogen atoms. This covalent bond is much stronger and more permanent when compared to the standard tertiary and quaternary interactions.
This is also the cause for experimental error in determining protein size/length for proteins with multiple subunits.
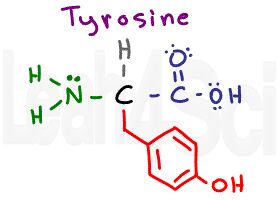 Tyrosine
Tyrosine
Tyr
Y
Some students see this as OH leaking from a tire (tyr).
Take a close look at tyrosine. What do you see? It looks like the aromatic phenylalanine with an OH group at the para position (ortho/meta/para 2nd video).
On the one hand, we have a very polar and hydrogen-bond capable OH group, but on the other hand, the OH is tiny when compared to the size of the benzyl group (CH2-phenyl). This conundrum is a common source of confusion, but if you understand this, you’ll recognize that tyrosine, while polar, is still a hydrophobic amino acid.
Polar Hydrophilic Amino Acids
Hydrophilic as the name implies comes from hydro-water and philic – loving.
Polarity comes from a 0.5-1.9 difference in electronegativity between bound atoms. While you don’t have to know these values for the MCAT, you should recognize that polar bonds will exist when N and O are bound to non-carbon atoms.
The electronegativity difference is enough to create a slight separation of charge or polarity. And since like attracts like, these partially charged groups will be attracted to oppositely charged or partially charged groups such as water. These groups will twist the polypeptide chain in order to interact with each other and with water.
Hydrophobic groups will twist away from these side chains.
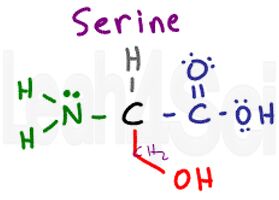 Serine
Serine
Ser
S
Think of serine as alanine with an OH group attached. Unlike tyrosine, the OH is the majority in this molecule and its polarity is enough to influence the entire group. This makes series polar and very hydrophilic.
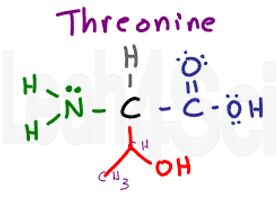 Threonine
Threonine
Thr
T
There are multiple ways to look at this group. You can think of it as serine with an extra methyl group, or as valine but with an OH replacing one of the methyl groups. I remember THREEonine as having 3 different groups: CH, CH3, and OH.
Like serine, this variable group is polar and hydrophilic.
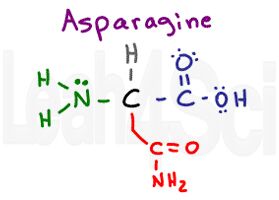 Asparagine
Asparagine
Asn
N
Try this: Slur your speech as you say ‘asparagine’ really fast. It sounds like you’re saying AS…N, which is how I remember the 3-letter abbreviation for this amino acid. The NH2 at the end of this molecule makes you think ‘base,’ but look at it’s neighbor. NH2 near a carbonyl forms an amide, which doesn’t like to act as an acid or base under standard physiological conditions. However, with partial charges and H-bonding capability at both the carbonyl oxygen AND the NH2 groups, we get a polar hydrophilic amino acid.
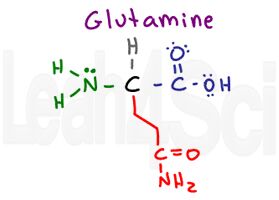 Glutamine
Glutamine
Gln
Q
I think of both ‘glut’ amino acids as gluttons having ‘consumed’ an extra CH2 group. Glutamine has the same structure as asparagine but with an extra gluttonous CH2 in its chain. Just like asparagine, it is polar and hydrophilic.
Acidic and Basic Amino Acid Side Chains
Acidity and basicity in amino acids is yet another source of confusion among students. If it starts out as an acid, does it become a base? How do I find the charge? And so on.
Here’s the trick: a carboxylic ACID in the side chain will give you an acidic amino acid. When a carboxyl group is deprotonated, you get a conjugate base SALT. So if you see the salt version of a carboxylic acid side chain, while it is TECHNICALLY a ‘conjugate base,’ we’ll simply refer to it as the salt version of the acidic amino acid. Same for the base. Look out for ‘non-distracted’ nitrogen atoms in the side chain.
Acidic Amino Acids
The acidic amino acids should look very familiar compared to asparagine and glutamine. And that’s because everything about them is the same except for the terminal functional group. Amides (discussed above) are polar, but if the NH2 is swapped for an OH group, you get an acidic carboxyl group.
Aspartic Acid / Aspartate
Asp
D
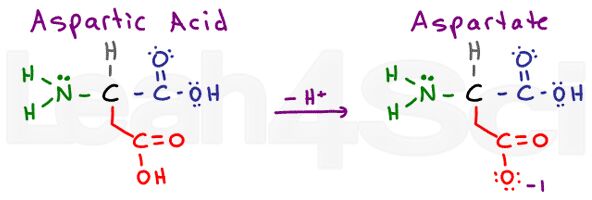
Aspartic acid refers to the protonated acidic form of the amino acid. When deprotonated, you’ll often see the conjugate base salt referred to as aspartate. This is the standard nomenclature for carboxylic acids.
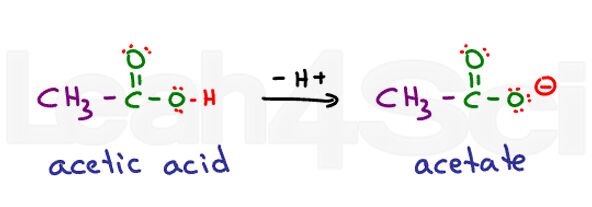
Think of ethanoic acid. Its common name is acetic acid. When deprotonated you get acetate. Acids are very stable in water since they are partially charged in their protonated form and fully charged in their deprotonated form. This makes them highly hydrophilic.
Glutamic Acid / Glutamate
Glu
E
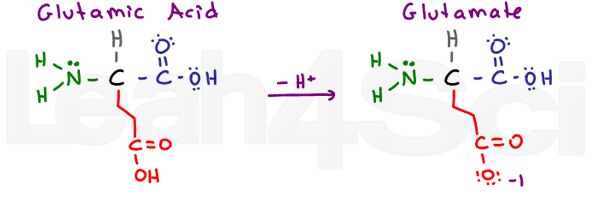
Once again we have a ‘glutton’ amino acid with an extra CH2 group. Glutamic Acid refers to the protonated acidic form, and glutamate refers to the deprotonated conjugate base/salt form.
Like aspartic acid, glutamic acid is very stable in water and thus hydrophilic.
Basic Amino Acids
Basic amino acids contain a nitrogen atom with a lone electron pair capable of attacking a hydrogen atom. When a basic amino acid is subjected to a low (acidic) pH, it will grab one of the free protons in solution to form a conjugate acid salt. These are easily recognize by the positive nitrogen in the side chain. Unlike the acidic amino acids, there are no ‘commonly used names’ to memorize for these conjugate salts.
Lysine
Lys
K
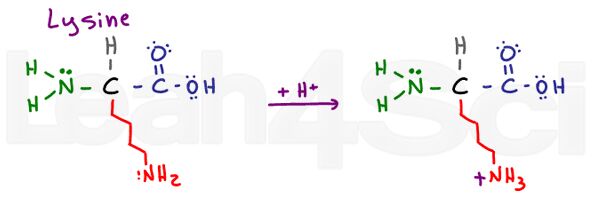
Lysine is a simple basic amino acid. Despite a long and potentially hydrophobic chain, it has a basic NH2 at the end. In its basic deprotonated form, lysine is neutral and hydrophilic; however, if found in physiological pH, lysine will pick up an H+ from solution to form an NH3+ salt. Salts are charged and therefore definitely hydrophilic
Arginine
Arg
R
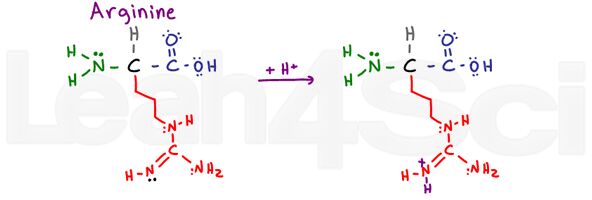
Arginine is confusing and makes me say ARGh or R for short. Why? The basic portion of this variable group consists of an NH, C=N-H and NH2.
The 2 single-bound nitrogen atoms can use their lone pairs to resonate with the carbon and double bound nitrogen atom. This makes their electrons UNAVAILABLE for acting as a base. However, the double-bound nitrogen uses its pi bond to resonate, leaving its free lone pair (shown in black) to act as the basic nitrogen on this group.
Argh!
Histidine
His
H
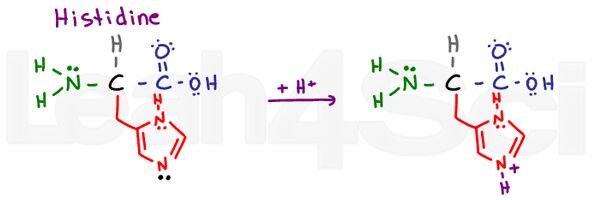
Histidine is another tricky base for the same reason as arginine. WHICH nitrogen is the basic one? Look at the drawing here, particularly at the lone pairs on the 2 nitrogen atoms. The histidine ring is a heterocyclic aromatic compound. The upper nitrogen atom does not have a pi bond. This means it must use its lone pairs to participate in resonance.
The lower nitrogen atom already has a resonating pi bond. This leaves its lone electrons (shown in black) free to grab a proton, making this the basic atom.
In conclusion
Amino acids are a critical component to biological structures and to your understand of biology and biochemistry on the MCAT. And so, as you attempt to memorize everything about these 20 amino acids, it’s important that you also understand why you have polar and nonpolar amino acids, what makes the variable group hydrophobic or hydrophilic, and of course, the logic behind protonated/deprotonated acidic and basic amino acids.