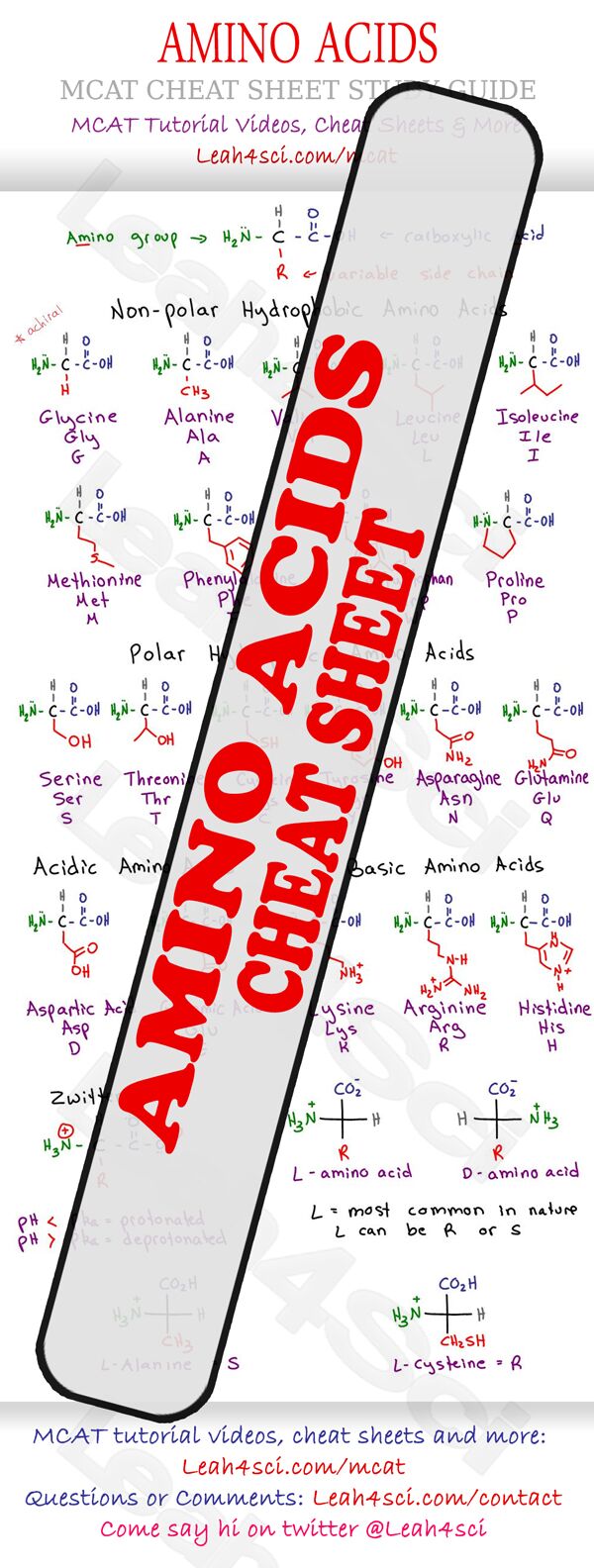
When it comes to amino acids on the MCAT you have to know… everything. But everything quickly gets overwhelming. And after a quick search, I didn't find any good amino acid charts so I've decided to create one for you. Download a copy to your computer and another to your phone.
When you find yourself waiting for the bus or in line at Starbucks, forget Facebook. Study this guide instead.
Click the image below to print/download the full version.
Ready to test what you know? Try my Amino Acids Practice Quiz, and don't forget to visit the Amino Acid Tutorial Video Series for full explanations, shortcuts, and more!




thanks
My pleasure!
like it … good job my English is not good as French but i try to read and uderstand
Thanks!
Thanks Leah!
You help me a lot, always. Please upload more videos and cheat sheets on biomolecules especially.
Thanks, once again.
Ninad: I try to record a new video at least once a week. Sometimes more, sometimes less 🙂
Hi! I’m a chem minor, and so this stuff is a little out of my league (even though I have to be able to decode it). How come there isn’t a specific name for a protonated glycine (zwitterion)? (Glycinic acid?)… The group I’m referring to is the second image below the “Finding Charge on Amino Acids in Preparation for Isoelectric Point Calculations” section.
Robert: The only amino acid names that change are the acidic ones. Aspartic Acid – Aspartate and Glutamic Acid – Glutamate.
hi leah, I shared your website, but I’m trying to download your amino acid and other cheat sheets. i received your email with the download link, but it doesn’t work; it keeps taking me back to your share page. will you provide the cheat sheet links that takes us directly to each cheat sheet?
Email me Avis
how come the histidine you drew have an extra H.
if histidne is close to ph 7 then in ph 7 shouldn’t it be a 1:1 ration?
why does C and Y have a hydrophobic region?
See the article explaining amino acids. https://leah4sci.com/understanding-amino-acid-side-chain-characteristics-for-the-mcat/
do you have to know pKa’s of each amino acid for the MCAT?
I would not. I’d know a general amino side chain, general carboxyl side-chain, acid/base and Histidine since it’s so close to physiological pH and therefor exists as a 10:1 ratio in many cases