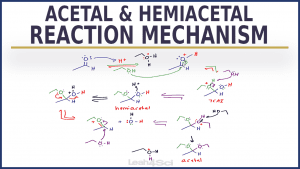
 Acetal and Hemiacetal mechanisms can be long, but when you UNDERSTAND each step you'll be saved from a big headache and trouble from merely memorizing.
Acetal and Hemiacetal mechanisms can be long, but when you UNDERSTAND each step you'll be saved from a big headache and trouble from merely memorizing.
This video walks you step-by-step through the acid catalyzed acetal formation reaction mechanism!
You'll see special attention on the acetal, hemiacetals, ketals, and hemiketals in the mechanism as well as resonance so you see exactly WHY each step is taking place. You'll also see the pattern so you can apply this to more mechanisms!
(Watch on YouTube: Acetal Mechanism. Click CC for transcription.)
<– Watch Previous Video: Acetal Ketal Hemiacetal Hemiketal Reactions Overview & Shortcuts
–> Watch Next Video: Cyclic Acetal Protecting Group Reaction and Mechanism
Links & Resources Mentioned In This Video:
This is Video 2 in the Acetal Series. Click HERE for the entire series.
Test your understanding with the Acetal Practice Quiz!



After the TCAI formation the Oxygen which get positive charge (O+) is attached with Hydrogen as well as Ethyl group which is a good electron donating group based on inductive effect. what I think madam is that positive oxygen can only attract more electron density from one of them, Hydrogen or Ethyl group
If it attracts more electron density from ethyl group then Hydrogen can not be the most positive atom, if it attracts more electron density from Hydrogen then; How Positive Oxygen can attract more electron from Hydrogen 1s orbital which is much closer to the nucleus than Carbon SP3 orbital which is away from the nucleus and as well as larger is size?
In that case ethanol could attack on carbon next to the positive oxygen.
kindly help, thank you.
Mdm Tay. Daughter in St Hilda’s Primary School. Jocelyn from TutorBee was very thorough when handling my requirements and prompt in following up. I needed an MOE teacher for my child and I was recommended one the next day. Still trying the tutor out but it has been so far so good.