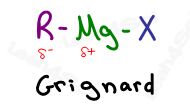
The Grignard reaction (pronounced Grin-yard) involves an R-Mg-X, a carbon chain bound to a magnesium halide, typically used to form alcohols by attacking carbonyls such as in aldehydes or ketones.
The Grignard reaction is my go-to for chain elongation in orgo 2 synthesis.
Alkynes are my go-to for orgo 1 chain elongation.
In English, please?
Let’s back up a bit.
Grignard Reagent Reactivity
Magnesium (Mg) is a Group II metal with very low electronegativity.
This is intensified by the electronegative halide, which gives it even more positive character.
Compared to magnesium, carbon has a much higher electronegativity, so that when bound to Mg, it behaves almost like a carbanion.
Carbanions are very reactive due to the unstable negative charge on carbon.
The R group can be any carbon chain including simple alkyl groups (no pi bonds) to more complex pi systems such as aryl or vinyl groups.
Watch the video below for an in-depth overview of Grignard Reactions, including activity, formation, reaction and mechanism.
Grignard Reagent Formation
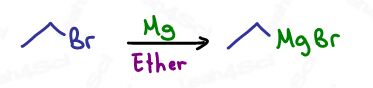
The Grignard reagent is formed by inserting magnesium into an alkyl halide, an R-X group.
Imagine the Mg squeezes itself in between the R group and the halogen.
The mechanism for this is quite complex, involving radical intermediates. Fortunately, this is NOT required at the undergrad orgo level.
What we DO need to focus on is the solvent.
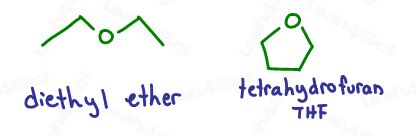
This reaction must be carried out in ether for two reasons:
- Ether stabilizes the Grignard compound.
- Ethers are not protic solvents, which would destroy the Grignard as explained in the video above.
You’ll typically see diethyl ether – Et2O, or Tetrahydrofuran – THF.
THF is a more appropriate solvent for aryl and vinyl halides, which are less reactive than alkyl halides.
Grignard Reactions
The Grignard is so useful in synthesis due to its high nucleophilicity. Remember, the carbanion in the Grignard is very unstable and very reactive.
They can be used in a variety of different reaction conditions.
I include a full list in the cheat sheet below.
You’ll see them most often used to attack a carbonyl to form an alcohol (early orgo 2).
Or attacking carboxylic acid derivatives (late orgo 2).
Grignard Mechanism
While the mechanism is unique for each reaction type, since many involve attacking a carbonyl, they typically follow the mechanism described below.
Key points to remember are as follows:
- The Mg-X is NOT reactive, but it MAKES the R group nucleophilic and very reactive.
- Carbonyl groups are capable of resonance with a partially negative oxygen and partially positive carbon. THIS is the electrophile in the reaction.
- The resulting alkoxide is negative and will require an acid workup to neutralize the final product.
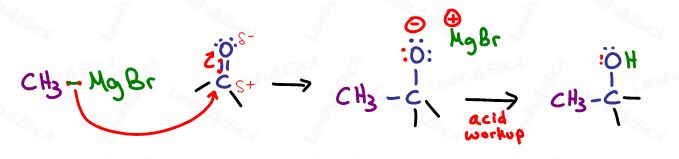
Let’s break it down
- The partially negative ‘carbanion-like’ carbon atom will use the C-Mg electrons to attack the partially positive carbon of the carbonyl.
- Carbon WOULD have 5 bonds violating its octet. The pi bond breaks as a result and collapses onto the oxygen as a negative lone pair.
- The remainder of the Grignard is now a positive Mg-X spectator ion in solution.
- We do an ‘acid workup’ to neutralize the final product.
Acid Workup Considerations
The final step requires a proton added to the negative oxygen. This is called ‘quenching’ or doing an ‘acid workup’.
You can show H+, H2O, or H3O+, or even just ‘acid workup’ for this step.
Make sure you clearly distinguish between step 1 Grignard and step 2 acid workup.
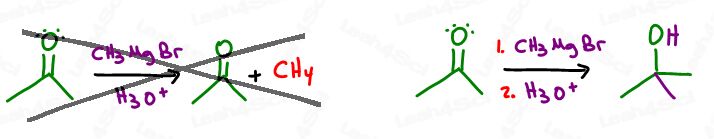
Why? If you add the acid workup BEFORE the Grignard attacks, the carbanion will attack the easier to reach and more acidic proton, rather than the less reactive carbonyl carbon.
This will destroy the Grignard and result in no reaction.
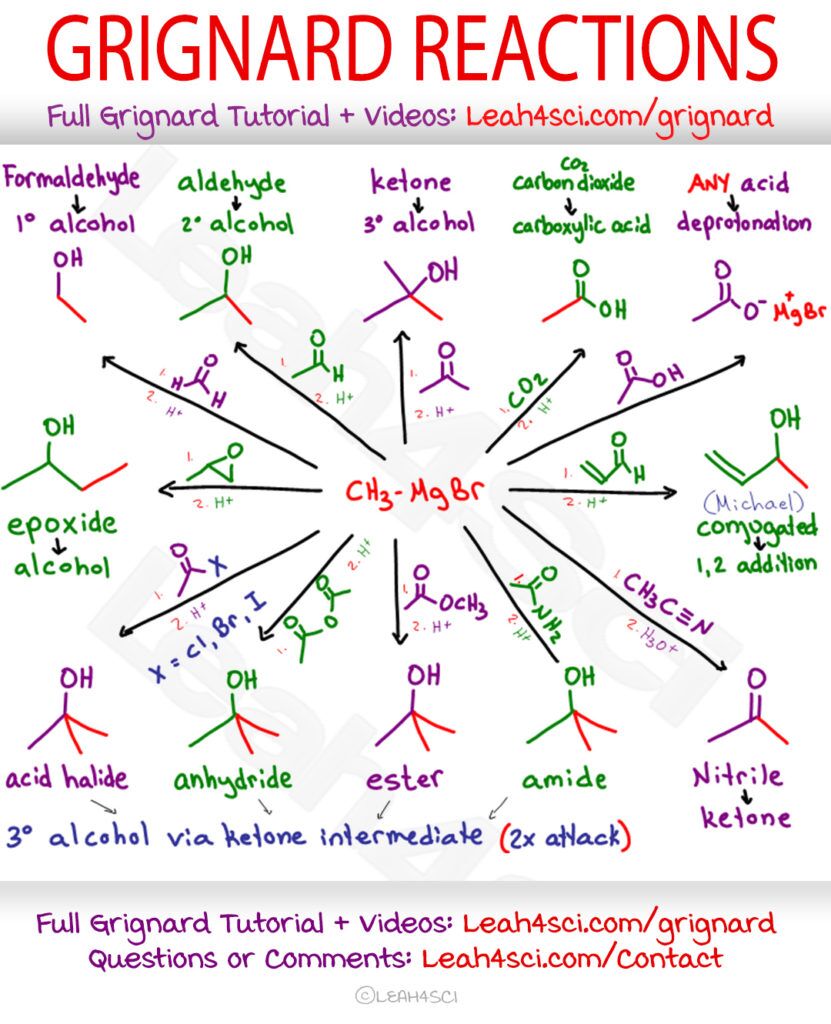
Grignard Reactions Cheat Sheet
Below is a cheat sheet list of the most common Grignard reactions you’ll come across at undergraduate level organic chemistry. Use the Active Writing method to help you memorize them.
Right-click the image to download, or Click to Download my entire Orgo Cheat Sheet PDF Collection.
Let’s talk through the most common reactions below.
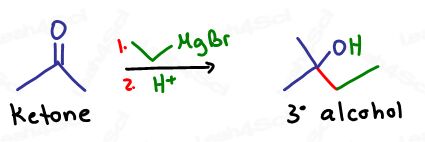
Grignards react with Aldehydes and Ketones to form Alcohols
When Grignards attack a carbonyl, the resulting product is an alcohol.
The type of carbonyl used determines the type of alcohol formed.
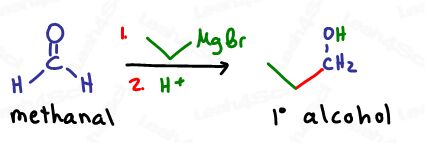
Primary alcohols are formed when Grignards attack methanal (formaldehyde), a one-carbon aldehyde.
Secondary alcohols are formed when Grignards attack longer chain aldehydes.
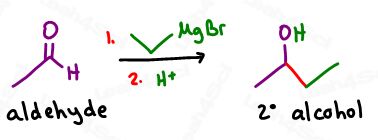
Tertiary alcohols are formed when Grignards attack ketones.
For a quick summary of the Grignard to Alcohol reactions as well as tips and shortcuts for predicting the products in organic synthesis questions, watch the video below.
Then, watch this video for a review on how to rank 1°, 2°, and 3° alcohols
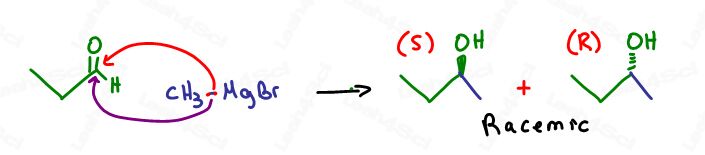
Stereochemistry of Grignard attack
Asymmetrical carbonyls may yield chiral products IF the attacking Grignard does not have the same R group as the carbonyl. However, any chiral products formed will be a racemic mixture due to starting with an sp2 hybridized carbon atom.
Sp2 carbons are trigonal planar (flat) and can be attacked from the top or bottom for different stereoisomers.
Carboxylic acid derivatives will yield varying results.
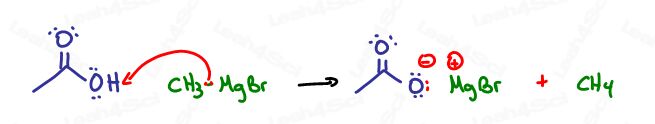
Grignards are destroyed in the presence of carboxylic acids.
While we can react them with derivatives, carboxylic acids are too acidic and will destroy the Grignard, just as the Grignard would attack any acid or polar protic solvent.
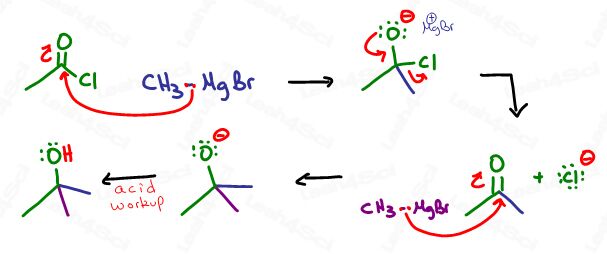
Carboxylic acid derivatives with good leavings will get attacked not once, but twice to form a tertiary alcohol via ketone intermediate. This includes the acid halide (acyl halide), acid anhydride and ester.
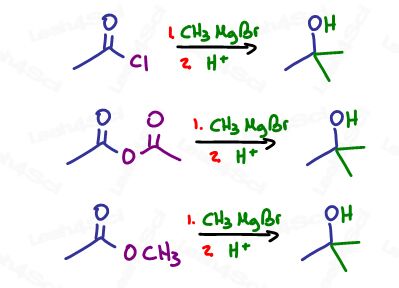
The reaction starts out with a Grignard attack on the carbonyl carbon forming an alkoxide anion.
With aldehydes and ketones we have nothing to kick out and so the negative remains until protonated.
Not so with a good leaving group. The negative oxygen attacks the carbon to reform the carbonyl pi bond (ketone), in the process kicking out the leaving group.
We already know that ketones are reactive to Grignards, which is why another one floating around in solution will quickly attack the ketone to form a tertiary alkoxide as demonstrated below:
Protecting Group Synthesis Note
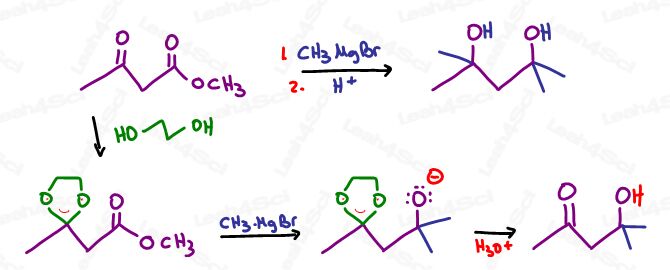
If carrying out a synthesis where you have more than 1 group susceptible to Grignard attack,
First, protect your second group before introducing the Grignard.
For example: protect your alcohol before attacking your ketone
Or use an acetal protecting group on ketones and aldehydes before attacking a carboxylic acid derivative.
Take a look at the reaction below, with and without an acetal protecting group.
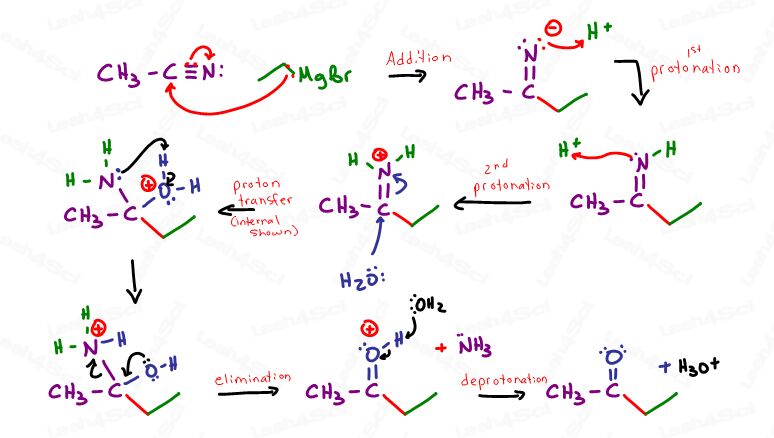
Grignards attack nitriles to form a ketone
This is a very different type of reaction, given the triple bond between C and N.
Following the Grignard attack, water will slowly displace nitrogen until it leaves as an NH3 with a ketone in its place, as demonstrated below in acidic conditions.
Note: This reaction can also take place in basic conditions for a different mechanism.
For a step-by-step breakdown of this reaction mechanism and to learn a shortcut for predicting the product of these kinds of reactions, make sure to watch below.
Other Common Grignard Reactions
Two more common Grignard reactions for your synthesis arsenal include chain elongation by attacking carbon dioxide and epoxide ring opening.
Chain Elongation
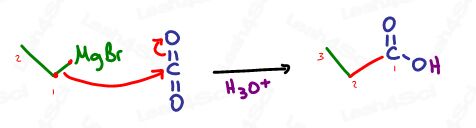
Grignards attack carbon dioxide, CO2 to form carboxylic acids.
If we can attack a carbonyl to form a single-bound oxygen, why not attack carbon dioxide as well? CO2 is simply 2 carbonyls in one.
Don’t forget, since CO2 contains a carbon, your final product will be a carboxylic acid with 1 more carbon than your attacking Grignard.
Grignards Open Epoxides (oxirane) at the Less Substituted Carbon
When it comes to epoxide ring opening (something I teach in-depth in the orgo study hall)
Grignards act like a nucleophile in an SN2 reaction.
They don’t wait for protonation, they have no carbocation intermediate steps, and they attack the less substituted, less hindered side
The resulting product once protonated has an alcohol,
But unlike our previous carbonyl reactions where incoming (Grignard) nucleophile and alcohol existed on the same carbon, in this case the nucleophile and alcohol end on adjacent carbons.
As with an Sn2 reaction, there is an inversion of configuration if chiral with the incoming nucleophile and alcohol anti to each other
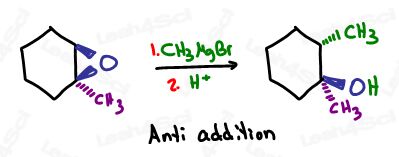
I realize this was a LOT to process for a single reagent. But hopefully, you understand just how important this is for chain elongation in synthesis and retrosynthesis.
Now that you understand the basics, take your time working through the reactions by following along with the Grignard Cheat Sheet.
Find the image above, or click to download my entire Orgo Cheat Sheet PDF Collection.
The Grignard reagent is just one of three common types of Organometallic reagents covered in Organic Chemistry courses. To learn about the differences between the Organometallics, as well as their reactions and uses in Organic synthesis questions, make sure to watch my video below on the Organometallics – Grignard, Gilman, and Organolithium.




Leave a Reply