 As an organic chemistry tutor, one of the first and more complicated topics that my students need help with is how to quickly recognize primary, secondary and tertiary carbon atoms within a molecule. There are a number of ways to do this.
As an organic chemistry tutor, one of the first and more complicated topics that my students need help with is how to quickly recognize primary, secondary and tertiary carbon atoms within a molecule. There are a number of ways to do this.
I’ll explain them then show you a trick you can apply to get your answer quickly on an exam. I call it the pencil trick.
After reading, be sure to watch the step-by-step Video Tutorial at the bottom of this page.
Overview of Primary Secondary and Tertiary Atoms
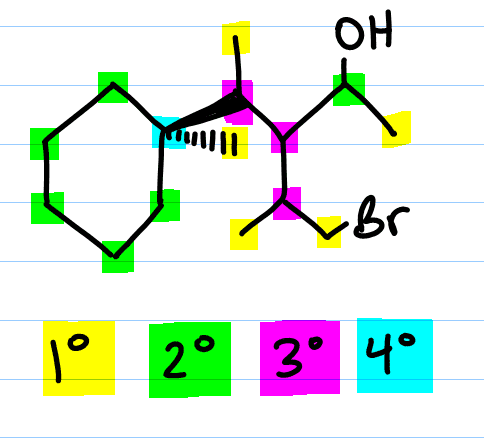
Primary, secondary or tertiary carbon refers to the number of carbons directly attached to the carbon in question.
 In other words:
In other words:
- A primary carbon can be written as 1° (#1 with a degree symbol) has one carbon attached to this carbon atom.
- A secondary carbon written as 2° (#2 with a degree symbol) is a carbon attached to two other carbons.
- A tertiary carbon written as 3° (#3 with a degree symbol) is a carbon attached to three other carbons.
- And a quaternary carbon written as 4° (#4 with a degree symbol) is a carbon attached to four other carbons.
I want you to recognize that the quaternary carbon is attached ONLY to carbon. This is because having four bonds to carbon means that carbon already has its complete octet. But primary, secondary and tertiary carbons will have other atoms attached. These atoms can include hydrogen, halogens (F, Cl, Br, I), oxygen, nitrogen, and so on.
Degree of Substitution
Degree of substitution means how many carbons are attached to the carbon atom in question. The answer would be something like primary, secondary or tertiary depending on what you have. The quickest way to recognize the degree of substitution is to look at the carbon in question and count how many carbon atoms stem from it.
When you have a molecule written in Lewis Structure it’s easy to see but when the molecule is written in line structure it can get tricky.
This is Where The Pencil Trick Comes In To Play
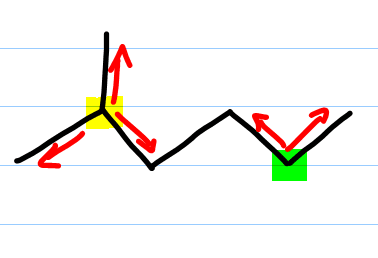
Here’s how to quickly recognize if a carbon atom is primary, secondary, tertiary or quaternary using the pencil trick. Identify the carbon in question and place your pencil on that carbon atom. Then examine how many lines emanate from the carbon atom that attach to another carbon—meaning the line does not lead to a hydrogen, oxygen or halogen. The number of lines that stem from that carbon represents the degree of substitution. Look back at the image above.
On the image I highlighted one carbon atom in yellow which, if you put your pencil on it, has three lines emanating from it making it a tertiary carbon. If you put your pencil on the other carbon atom, highlighted in green, you’ll see two lines stemming from it, making this a secondary carbon.
Alcohol and Halogen Substitution
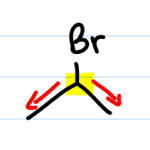
When referring to the degree of substitution of a halogen or an alcohol, you don’t look at the halogen or alcohol itself but rather at the carbon holding it.
So for example, if you look at bromine in the molecule drawn here, you’ll see that bromine is attached to one carbon. But this bromine is not a primary halogen even though it’s attached to one carbon. You must look at the carbon instead. Do the pencil trick and see that this carbon is attached to two other carbons. Since the carbon is secondary, so is the bromine.
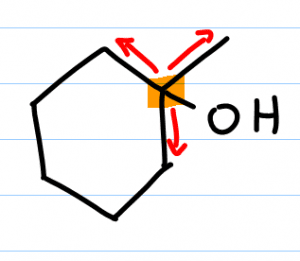
The same thing applies for alcohol. For example, if the alcohol is on the tertiary carbon then the alcohol is considered tertiary; not because it’s attached to one carbon but because the carbon holding the alcohol is attached to three other carbons.
Amines (Nitrogen) Are Classified Differently
Degree of substitution for amines is slightly different than alcohols and halogens because in this case we’re actually looking at the nitrogen atom.
A primary amine is one that has the nitrogen or an NH2 group directly attached to one carbon. We don’t care if this is a primary, secondary or tertiary carbon because it’s the nitrogen that we’re examining.
A secondary amine is an NH group attached to two other carbons.
A tertiary amine is a nitrogen attached to three other carbons.
Sometimes you'll see an ammonium salt, which is a positive nitrogen attached to four different carbons.
The reason I said NH2, NH and N for primary, secondary and tertiary is because we’re assuming our amines are neutral. If nitrogen can have a total of three bonds and one lone pair, we expect that when nitrogen is bound to one carbon, it will have two hydrogens.
When nitrogen is attached to two carbons it will have one hydrogen.
When nitrogen is attached to three carbons it will not have hydrogen.
That’s why ammonium is the different one, the positive nitrogen has 4 bonds and no lone pairs.
You can actually apply the pencil trick to nitrogen as well. Just put your pencil on nitrogen and see how many carbons stem from it.
(Watch on YouTube: Pencil Trick. Click cc for transcription.)



Would an alcohol attached to a benzene (phenol) be considered a secondary or tertiary alcohol? The OH is attached to only 2 other carbons, but it has 3 bonds, granted one is a pi bond. So, as with all groups in question theatre attached to an sp2 carbon, are they secondary or tertiary. Similarly, one could ask: is a group that’s attached to an sp carbon (alkyne) be considered a primary or tertiary group?
The explanation are awesome. Thank you mam.
what about a double bond in the ring
Hey Leah on the Morphin example when you are counting the secondary C do we not take account the bonds found within the aromatic ring?
Nice trick……….Thanks
I loved it..thank you
whats the difference in CH3 and H3C and why do you use both forms?
If there is a double or triple bond between any two carbons…?? Then….
love,love,love it thanks leah!!!
THANK YOU !!!!
Mam
How do you classify Hydrogen atoms as Primary secondary or tertiary?
I always get muddled up in that. Any tips?
Hi
I have a question
Plz tell me how many primary carbon atoms are present in 1-propyne?
I love your technique in explaning that topic.
Hope to learn more from you..
Thanks for the nice, simple yet very effective explanation – it helped me a lot coming quickly to a good understanding of the difference between primary, secondary and tertiary amines and the difference in nomenclature between amines and other functional groups. Thanks much!
Ya solved my query quite smoothly……thank. can ya tell tricks for other things too?????
Thank you.. Helpful trick solving these type of question is now easier with the help of your trick.
Thanks Leah, very useful. Pencil trick is excellent. U gave me clarity about primary, secondary, tertiary.
God bless you 🙂
I don’t understand how you identify the carbon in question, especially if, for instance, you have a kekule structure with a cabon in the center, three CH3’s attached to the center carbon, and NH2 also attached to the center carbon. I want to call this tertiary because the central carbon is attached to three carbons, but it is incorrect and I don’t understand why.
What do you do in the case of a double bond?.. let’s say, in a cyclohexane? How would you count the carbons?
Thank you so much ma’am…..the trick is really helpful.
Thank u so much , it really very helpful to me . Thanks a lot
This summer I am prepping for Org 1. I have not had the opportunity to apply your teaching formally. I would rate this particular topic explanation very good. I think you could improve upon the very good topic link by: 1. I spent 3 min studying the first figure, then the wording made sense. I think if you put emphasis/expanded on the initial carbon and the first degree, by elaborating that the carbon itself is “connected” to itself, and itself only, which by default makes it primary, would have helped, similar to how you started your explanation of the quaternary. 2. I think you should have kept your color coding consistent by marking the alcohol with purple instead of orange. 3. I think you should have included a figure in your great teaching of the nitrogen(amine) group. Thanks for creating a great website.
if there is a double bond between 2 carbons, for example in ethene (H2-C doublebond C-H2)
Are both the carbons still primary or they are secondry?
Do bonds matter in defining the primary, sec.,and tert. carbons?
On top of working full time, being a mom, going to school full time, and doing a program at Stanford University twice a month, you give me SO much hope! Freakin’ adore you and this pencil trick! My day just got 100{4de72ea05502f43f582be05109791d04c2e06ba4d55e604218cbe1ea315cd788} better. Binge watching O’Chem videos this weekend! You are loved. Thank you for all you do.
That was really helpful
It is amazing. Can you explain about hybridisation???
Great trick
I want you to know that i’m so grateful for the effort you put in. I have dyslexia and studying medicine and it’s thanks to people like you i can manage the studies
I still dont get it. But leah4sci girl in pic is so cute
success is depend on your education if you have good education with degrees success is not far for you but if you have only experience but not education equal to your experience you never get success for those people online universities offers buy a degree online with transcript equal to your experience.
Thats really an Amazing trick… It would be great if you give some more examples!
huh please help i still don’t get it
Marga, can you be more specific about what you don’t understand?
It’s simple…go back and watch it again
Thank you, this is SO helpful! Such a simple idea that really works. Finally something ‘simple’ in orgo! 🙂
You’re welcome! Glad it clicked for you!
you are really the best
Thanks, Armin!
so do you refer to an ammonium salt with 4 carbons as Quaternary?
trick looks pretty like you only sister
itz amazing i love it…………..:)
Jithendra, uh… thanks?
HELPED A LOT! THANK YOU SO MUCH ..
You’re very welcome Yumna
Thx soooo much
You’re welcome Jui
Thank you
Thanks Priyanka
you are a legend miss
you are the greatest teacher of my entire life
respects
Thanks Naseer
do u have any video that explaining above stuff? cause i dont really get it
Sorry, no
This s jst super cool…dis helped me a lottt!!! Guys…..jst follow dis ull…find d soln for ua prob…!! All d bst!!
This makes so much since how you explained it! I watched your other videos for Organic Chem (I’m in O. Chem 1 right now) and it helped me so much when I was studying for my midterm I had the other day. Thank you!
That’s awesome Marissa! I hope you aced your midterm
Thanks for sharing best tips for using of pencil tricks and i hope you regular give us the great information.
You’re welcome! I have a TON of resources on the site so be sure to use the search bar to find them
Thanks Leah. You are great.
You’re welcoem Pankaj
thank you Leah. you are great so much easier
You are very welcome Ruth
Hey, that’s a really simple but EFFECTIVE way to figure out primary, secondary, and tertiary…thanks Leah!!
Glad you find it helpful Russ. If you’re allowed a highlighter on exams it’s even easier
Very good trick Leah,.. easy to visualize it… thanks!
Thank you Damarys. I love this trick because it’s so ‘childish’ and fun, yet works at the college level. I’m a kid at heart so… 🙂
organic is soo easy lit my favourite subject. i dont understand why so many people complain about orgo.
Organic is a fun topic, but not that easy to get compared to the other sciences. Since most students don’t get it right away, and don’t always have the best resources available, they complain…
Awesome, glad I can help. What else did you get in addition to the pencil trick?
I learnt to actually reCognise the primary secondary tetiary and quatenary carbon n NItrogEn
Awesome! Nitrogen tends to throw off lots of students because of the way the carbon atoms are bound/counted. What are you studying right now?
I’m a first year Pharmacy student………
Very cool, lots more organic chemistry coming your way 🙂
Thanks Leah really helpful
You are very welcome Elvira. What did you like most about this trick?