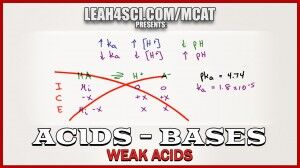
 Ka and pH calculations for weak acids can be trick on the MCAT if you attempt a general chemistry approach.
Ka and pH calculations for weak acids can be trick on the MCAT if you attempt a general chemistry approach.
Ice tables and quadratic equations are not only a waste of time, but nearly impossible without a calculator.
This video shows you my shortcut for skipping the ICE chart and skipping the quadratic equation for weak acid calculations.
(click to watch on YouTube. Read Video transcript here)
Resources mentioned in this video:
- pH/log math shortcut trick tutorial video
- Decimal shortcut trick tutorial video
See Previous Video: pH and pOH Calculations for Strong Bases in MCAT Chemistry
See Next Video: pH pOH ka and kb Calculations for Weak Bases in MCAT Chemistry
This is video 4 in the MCAT Acid Base series. Catch the entire series HERE



What if I am given the pKa instead of Ka? For example, just given acetic acid pKa = 4.74. How do I quickly convert this into Ka so I can use that to find the pH?
What if you have the H+ or A- and you’re trying to find the HA, would it still be Ka= x^2/Mi, but just rearranging the equation?