Understanding chirality is important, not only to organic chemistry, but to understand the world around you in general. I like to think of chirality as ‘handedness’.
Understanding chirality is important, not only to organic chemistry, but to understand the world around you in general. I like to think of chirality as ‘handedness’.

We have a right and left hand that are similar to each other, mirror images in fact. Yet our hands are not super-imposable. A right glove will not fit a left hand due to the opposite ‘handedness’.
Be sure to check out the Optical Activity Mini Series in Videos 9-11 below!
As you work through this series, pause and try each question and make sure you’ve mastered a topic before moving on.
Download my free Stereochemistry Cheat Sheet to follow along, and be sure to try the Stereochemistry Practice Quiz once you feel ready.
Also, do you need a Model Kit? Watch How to Use Your Organic Chemistry Model Kit.
 Video 1 – Introduction to Stereochemistry, Enantiomers and Chiral Molecules
Video 1 – Introduction to Stereochemistry, Enantiomers and Chiral Molecules
Let’s start at the very beginning. WHAT is chirality? What does it mean for molecules? How can you identify a chiral carbon and what does that mean for its non-superimposable mirror image? All of this and more
 Video 2 – R and S Configurations using Cahn-Ingold-Prelog rules
Video 2 – R and S Configurations using Cahn-Ingold-Prelog rules
Now that you understand about chirality, how do you find it’s absolute configuration to see if it’s R or S? Learn how to rank substituents using the Cahn-Ingold-Prelog system and using priorities to differentiate between R and S stereoisomers.
Video 3 – Finding R and S When Group 4 is Forward
Finding R and S is easiest when priority group #4 is towards the back. But how do you find if a molecule is R or S when group #4 is facing forward? More importantly WITHOUT having to visualize a confusing 3-dimensional molecule? Learn how to use my ‘think backwards method’ and how I learned this in the military by watching video 3
 Video 4 – Swap Method For Finding R&S Configurations
Video 4 – Swap Method For Finding R&S Configurations
Swap Method Trick for quickly finding R and S configurations when group #4 not forward/back. Save time AND forget your model kit and mental molecule manipulations redrawing structures on your exam.
 Video 5 – R & S Configuration for Lengthy Complex Substituents
Video 5 – R & S Configuration for Lengthy Complex Substituents
This orgo tutorial video shows you how to approach lengthy and complex substituents so that you can quickly and easily determine R and S configurations.
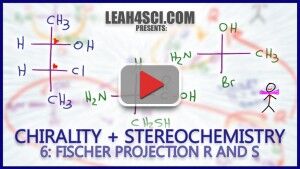 Video 6 – Fischer Projection Stereochemistry Trick
Video 6 – Fischer Projection Stereochemistry Trick
This video shows you how to quickly and easily find R and S configurations directly on the Fischer Projection. Guaranteed to save you time on your next stereochemistry quiz/exam.
 Video 7 – Newman Projection Stereochemistry R&S Trick
Video 7 – Newman Projection Stereochemistry R&S Trick
This video teaches you how to quickly find configurations of molecules presented in a Newman Projection. Learn how to view the molecule and quickly assign priority WITHOUT the need to redraw into a sawhorse projection.
Video 8 – Diastereomers and Meso Compounds: Multiple Chiral Centers
This video shows how to quickly identify enantiomers, diastereomers and meso compounds in chiral molecules with more than one chiral center using R and S configuration and the swap method. Learn how to use an Organic Chemistry Model Kit to visualize the plane of symmetry in meso compounds.
◊ Optical Activity in Stereochemistry Mini-Series ◊
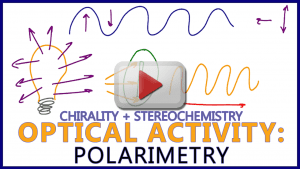 Video 9: Polarimetry
Video 9: Polarimetry
Each enantiomer of a chiral molecule has its own characteristic of light rotation! And not to be confused with R/S!!
This video introduces polarimetry, racemic mixtures vs pure solutions, +/- , dextrorotary (D) and levorotary (L), and more.
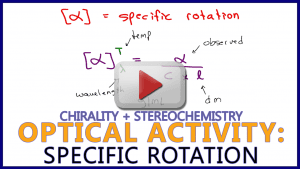
Video 10: Specific Rotation
This video details the factors influencing specific rotation such as observed rotation, g/mL, path-length, wavelength, and temperature. Along with test-level examples!
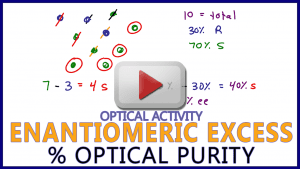
Video 11: Enantiomeric Excess Percentage and Optical Purity
This video shows the logic behind EE% or Enantiomeric Excess Percentage, also known as Optical Purity Percentage. Step by step example calculations are shown and more.
Don't forget to Download my free Stereochemistry Cheat Sheet to remember all the details and be sure to try the Stereochemistry Practice Quiz to test your skills!