Aromaticity is one of the more exciting topics in organic chemistry, typically covered right before Electrophilic Aromatic Substitution. The concept can be tricky but the rules are relatively straightforward. Once you master this topic, aromatic questions become the source of quick and easy points, on an otherwise more difficult exam.
In this tutorial I will attempt to break it down and show you just how simple and quickly you can solve questions on these arene compounds. Typical examples are benzene and toluene.
We’ll start out with the basics to understand the criteria for aromaticity. We’ll then focus on differentiating between aromatic, non-aromatic, and anti-aromatic.
Once you get the basics we’ll look at the trickier questions including fused rings, anion and cation aromaticity followed by heterocyclic compounds.
Take your time working through this tutorial and make sure you master each aspect before moving forward by taking the aromaticity practice quiz!
For a reference of some of the most common aromatic compounds, don't forget to download my Common Aromatic Compounds cheat sheet.
So what IS aromaticity?
Derived from the word aroma, the earliest compounds studied had a pleasant smell, hence the name ‘aromatic’. However, some aromatic compounds are odorless.
Aromatic compounds are very stable due to resonance stability of the conjugated electrons and overlapping pi bonds.
What does this mean for organic chemistry students?
4 Criteria for Determining Aromaticity
Look for the following 4 criteria to identify aromatic compounds. I like to write a quick ‘checklist’ next to the molecule, or do a mental checklist when pressed for time.
 4 checks = aromatic
4 checks = aromatic
- Cyclic
- Planar
- Conjugated
- Huckel’s Rule
Watch the video to preview concepts, or scroll down for detailed written/draw explanations.
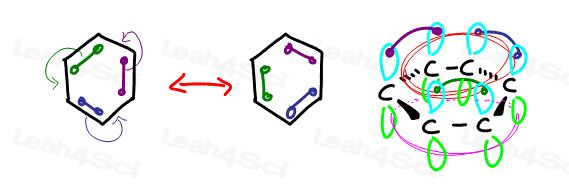
Cyclic
Cycle or cyclic implies a ring. A cyclic molecule is one in which the first and last carbon in the chain are bound to each other. (See the Naming Cyclic Compounds for review)
Carbon-1 is attached to carbon-2 but also attached to C-6
In hexane C-1 and C-6 are not attached to each other making it a linear compound
Planar
Since aromaticity relies on the ability of orbitals to overlap, the ring must have its atoms in the same plane.
In simple English?
The molecule must be flat.
What does this mean for hybridization?
Recall that sp3 carbons are tetrahedral or 3-dimensional
sp2 carbons are trigonal planar or simply ‘flat’
Rusty on hybridization and geometry? Review Sp³, sp² and sp Hybridization (+videos)
Conjugated
You may have memorized that conjugated systems have alternating single and double bonds. This is true but not as intuitive.
Instead look for consecutive sp2 atoms capable of resonance.
Remember this rule:
Never Ever Ever Ever Resonate Onto an sp3 Carbon Atom!
A conjugated molecule has its electrons distributed over the entire molecule, in this case over the entire ring. If there is an atom in the ring NOT capable of resonance it destroys the ‘cyclic flow of electrons’ making it not fully conjugated.
Huckel’s Rule
This is potentially the trickiest criteria on the checklist, unless you
- Fully understand it
- Learn my shortcut
Before we apply the logic, let’s get this out of the way:
The true science behind huckle’s rule deals with molecular orbital stability and whether a planar ring molecule will have aromatic properties… This is beyond the scope of what is covered in an average undergraduate organic chemistry course.
Without going into the technical details we’ll simply focus on the outcome of his equation.
Huckel’s Rule: 4n+2 = Number of Resonating Electrons
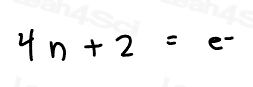
Resonating electrons include both pi electrons and lone pairs.
When solving for ‘n’, n must equal to a whole number.
If we get a fraction then the molecule DOES NOT obey Huckel’s rule.
Benzene is the most common aromatic molecule. a benzene ring has 3 pi bonds thus 6 resonating electrons.
4n+2 = 6
Solve for n
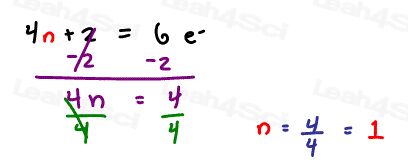
n = 1 which is a whole number.
Benzene follows Huckel’s rule.
Since benzene also follows the previous criteria it is indeed aromatic.
Huckel’s Rule Trick
While the concept and math are important for LEARNING, it’s a waste of time on your orgo exam.
Once you’ve mastered the math above, take a look at these patterns
Solve 4n+2 = 4 pi electrons
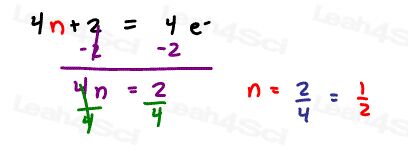
Solve 4n+2 = 8 pi electrons
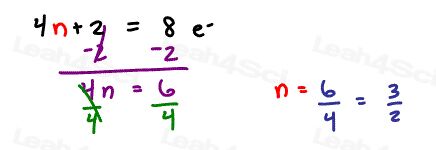
Now solve 4n+2 = 10 pi electrons
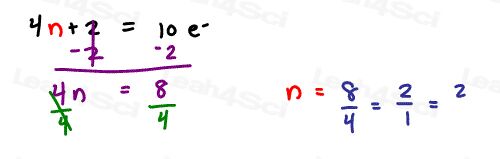
Are you noticing a pattern?
4 and 8 yield fractions, 6 (benzene) and 10 yield whole numbers. Every other even number should yield a whole number for ‘n'.
What if we continue this pattern?

The pattern tells us that 18 should yield a whole number
Let’s prove it
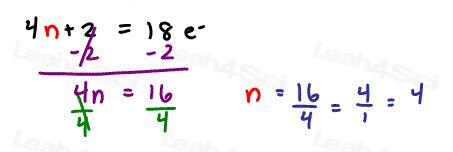
These numbers look at JUST the number of electrons. But electrons are paired. Look at what happens when we count in pairs?

Notice something?
Odd number of pairs follow Huckel’s Rule, even pairs do not!
Don’t take my word for it. ONLY once you’ve PROVEN THIS TO YOURSELF should you memorize the odd/even pattern and save yourself precious minutes on your next exam.
What happens if a molecule does NOT follow all 4 criteria?
Your exams will likely present a handful of molecules and ask you to classify them as follows:
- Aromatic
- Anti-Aromatic
- Non-Aromatic
Aromatic is simple, it follows all 4 criteria above.
The difference between AntiAromatic and NonAromatic is the cause for many lost quiz/exam points.
Let’s explain this with a story.
NY has 2 Major League baseball teams. Yankees and the Mets.
I’m a big Mets fan.

Mets fans joke that we support 2 baseball teams:
- The NY Mets
- Any team that beats the Yankees
I bet Yankee fans feel the exact opposite, especially after the Mets made it to the 2015 World Series.
If we compare a hard core Yankee and Mets fan, you’ll see most the same criteria. Everything baseball related will be the same.
- Deep understanding of the game
- Watch most games on TV
- Attend a few live if possible
- Annoy friends with baseball details
- Lots of Team paraphernalia including caps, t-shirts and more
So what is the DIFFERENCE between a Mets and Yankee fan?
Everything above is the same!
It’s that ONE crucial factor, the very team we root for.
I clearly root for the Mets.
Now let’s take my sister as a counter example. I think she MAY have attended ONE baseball game in her life. She doesn’t fully understand the game and frankly, she doesn’t care.
Curveball vs knuckleball? Ground out vs foul out?
It makes zero difference to her and I doubt she even knows what they are.
She’s not FOR baseball. She’s not against or ANTI baseball.
She probably doesn’t even know what the Subway Series (Mets vs Yankees) are all about.
She’s a Non-baseball fan who doesn’t care one way or the other.
Back to Aromaticity
Aromatic and Anti-Aromatic compounds are nearly the same!
They follow all the criteria and look THE SAME to an outsider. They simply root for a different team.
Aromatics root for Huckel’s rule so that n = whole number
Anti-Aromatic root for the opposite team so that n = fraction
Non-Aromatic compounds are like my sister.
They don’t care about aromaticity.
They don’t care to follow cyclic, planar, or conjugated.
They MAY follow 1 or 2 out of 3 ‘by accident’ but not because they’re trying to be fans of aromaticity
In Simple Terms:
Aromatic: cyclic, planar, conjugated, Huckels’ Rule
Anti-Aromatic: Cyclic, planar, conjugated, DOES NOT follow Huckel’s Rule
Non-Aromatic: Missing at least ONE of the first 3 criteria
Let’s try a few examples before you jump to the common aromatic compounds cheat sheet
Take a look at 1,3-cyclobutadiene
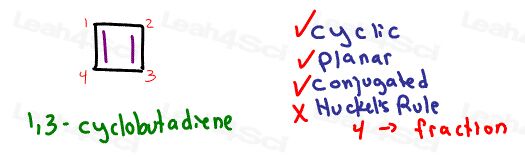
It’s cyclic, planar, conjugated, but has a total of 2 pi bonds or 4 resonating electrons.
It’s clearly a fan of the ‘aromaticity’ concept, but is ANTI Huckel’s Rule and therefore Anti aromatic
Now let’s take a look at trans-3-hexene.
This is an obvious example
It’s not cyclic
It’s not planar
It’s not conjugated
Clearly not interested in aromaticity making it Non-Aromatic
Another fun tricky example: 1,3,5-hexatriene
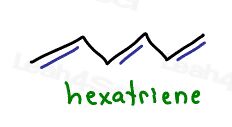
It’s not cyclic
but it does follow the rest
It’s planar, conjugated, and appears to follow Huckel’s rule with a total of 6 pi electrons for an odd number of electron pairs
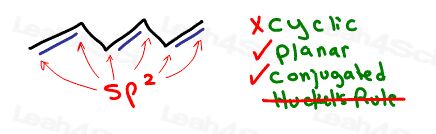
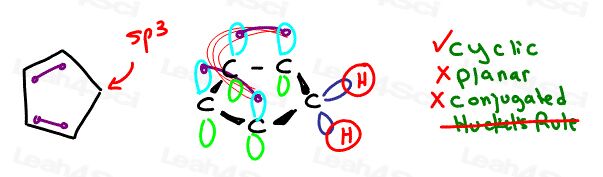
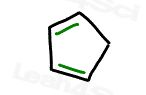 Let’s analyze 1,3-cyclopentadiene as a less obvious example.
Let’s analyze 1,3-cyclopentadiene as a less obvious example.
It’s cyclic, it appears partially conjugated, but it’s not planar.
Having an sp3 tetrahedral carbon in the ring makes the ring 3-dimensional.
The sp3 carbon also prevents full resonance around the ring.
We don’t even bother with Huckel’s rule because we’ve immediately ruled it out on the earlier criteria
Tricky Exceptions to Aromaticity
Sometimes a molecule may APPEAR to follow the checklist, but something unknown will alter its physical appearance. Professors love to trick you with these so memorize it!
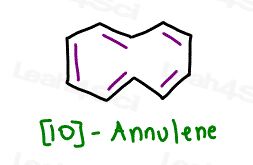 Here’s a fun one to consider: [10]-annulene
Here’s a fun one to consider: [10]-annulene
It appears cyclic
it appears planar
it appears conjugated
it follows Huckle’s Rule
But… what about steric hindrance?
[10]-annulene is in fact NOT a planar molecule despite having all sp2 carbon atoms.
Look at the carbon atoms depicted in red 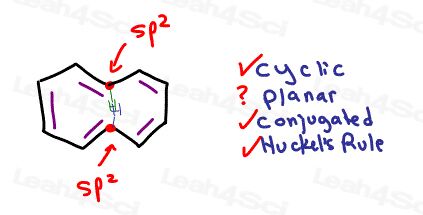
If each carbon has bonds at 120 degrees then the blue and green hydrogen atoms would occupy the same space.
This can’t happen!
[10]-annulene will twist slightly so that one hydrogen can shift up and the other down. The attached carbons are dragged along. This contortion makes the entire molecule non-planar making it NON-Aromatic.
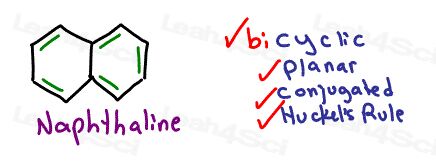
Polycyclic Aromatic Compounds
Aromatic compounds are cyclic. But the aromaticity is not limited to a single ring. In fact, you may see many fused rings participating in a large aromatic structure.
The key is to determine if the entire system still follows our simple checklist.
Recall from above that [10]-annulene is non-aromatic due to the warping and 3-D structure of the…
If we replace the 2 hydrogen atoms with a bond between carbons we form Naphthalene. 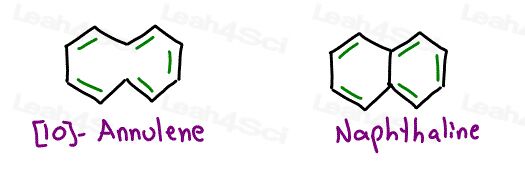
Naphthalene has the appearance of 2 fused benzene rings and does follow all the rules.
We count the pi bonds in both rings for a total of 10 electrons for an odd number (5) of electron pairs.
Charged Aromatic Compounds
Don’t let charged compounds confuse you. The same rules apply for anions and cations.
Simply draw the checklist and see if the molecule matches all 4 criteria.
The key to keep in mind is this:
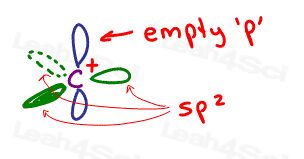
- Carbocations are sp2 hybridized with an empty p-orbital capable of accepting a resonating pair of electrons
- Lone pairs of electrons CAN inhabit a p-orbital and resonate with neighboring pi bonds
Aromatic Cations
 The word Cation comes from Cat = positive, and ion = charged atom/molecule
The word Cation comes from Cat = positive, and ion = charged atom/molecule
carbocations are positively charged carbon atoms.
This comes from carbon having 3 bonds and no electron pairs. carbocations are sp2 hybridized with 120 degree bond angles for trigonal planar (flat) geometry.
There IS a perpendicular empty p-orbital capable of accepting resonating electrons.
Look at this tiny 3-membered ring.
While there is a great amount of ring strain, this molecule IS considered stable.
Why?
It’s cyclic
It’s planar
It’s conjugated
It has a total of 2 resonating electrons for an odd number of electron pairs (1) making it aromatic

Now take a look at the cyclopentadienyl cation
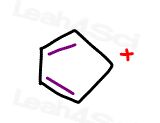 It’s cyclic
It’s cyclic
It’s planar
It’s conjugated
But with only 4 resonating electrons we have an even number of pi bonds disobeying Huckel’s Rule making it anti-aromatic
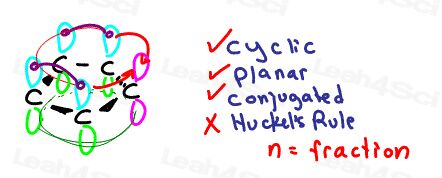
Aromatic anions
Anions are An = negative, ion = charged
Anions have an added lone electron pair carrying a negative charge. If placed next to an sp2 hybridized atom such as a pi bond, the negative electrons CAN resonate and join a conjugated system.
Let’s take a look at the cyclopentadienyl anion
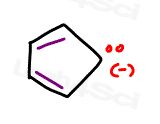 It does not appear to complete the ring of resonance.
It does not appear to complete the ring of resonance.
But unlike its positive counterpart above, this anion contributes an extra lone pair to the system.
This molecule is cyclic, planar, conjugated, and follows Huckel’s Rule.
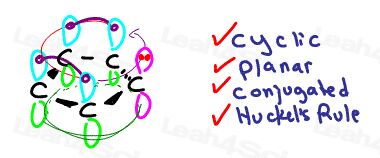
It’s aromatic
Let’s contrast with a 6-membered anion 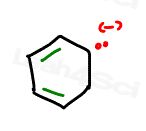
This appears to follow all the rules again.
However, the anion carries 2 electrons on just one carbon atom. Yes this is capable of resonating with the pi bonds.
But take a look at that sneaky sp3 hybridized carbon atom which has 2 hydrogen atoms completing its octet and preventing any resonance from coming its way.

This molecule is NON-aromatic since it violates at least one of the first 3 criteria
Heterocyclic Aromatic Compounds
The term heterocyclic comes from Hetero = different, and Cyclic = ring.
Heterocyclic molecules have atoms other than carbon in their ring. You’ll commonly see oxygen, nitrogen, and even sulfur in heterocyclic aromatic compounds.
Heterocyclic molecules can also participate in aromaticity. This is evident in many biological molecules from amino acid side chains to the Nitrogenous bases on DNA and RNA
If the atom is NOT carbon, don’t worry about it. Treat it just as you would a carbon atom and follow the checklist.
The tricky aspect of heterocyclic compounds arises with lone pairs.
To use them, or NOT to use them?
That is the question
Let’s take a look at pyrrole and pyridine
They’re each cyclic, planar, fully conjugated, and yes, they EACH have 6 resonating electrons.
The difference?
Pyrrole uses its lone pair in resonance, pyridine does not.
So how do you know if to count an atom’s lone pair or not?
NO you don’t look to see if the electrons are drawn in or out of the ring.
That’s arbitrary.
This is a tricky question with a very simple answer.
Let’s assume the atom WANTS to participate in aromaticity.
Ask yourself this question:
“Can the O, N, S, B… atom participate in resonance as is, or is it required to bring its own electrons?”
If the non-Carbon atom already has a pi bond, meaning it’s already participating in resonance DESPITE considering it’s lone pair, ignore its lone pair as seen in pyridine.
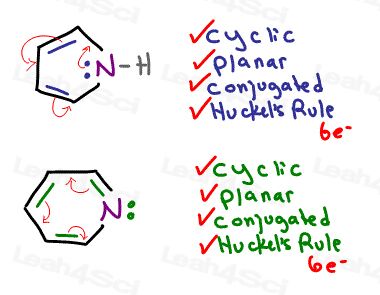
If the atom is not able to participate UNLESS it supplies its own electrons, it must provide its own electrons to participate in resonance.
In other words,
Use the atom’s own electrons WHEN YOU NEED THEM.
This is even more exciting with a molecule like thiophene 
Thiophene is 5-membered heterocycle with a sulfur atom.
Sulfur has 2 lone pairs of electrons.
In order to participate it must bring its electrons, but it only requires ONE pair, and so the other electron pair is left out.
This gives us a total of 6 resonating electrons allowing it to complete the checklist
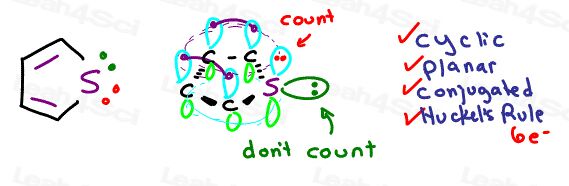
Thiophene is aromatic
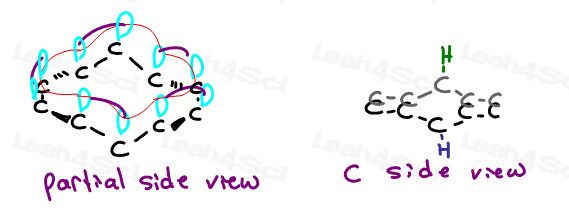
The Technical Explanation of the Heterocycle Electron Trick
A good orgo mnemonic or trick is only valuable IF you understand the logic behind it.
Let’s take a side view for each of the heterocyclic compounds analyzed above.
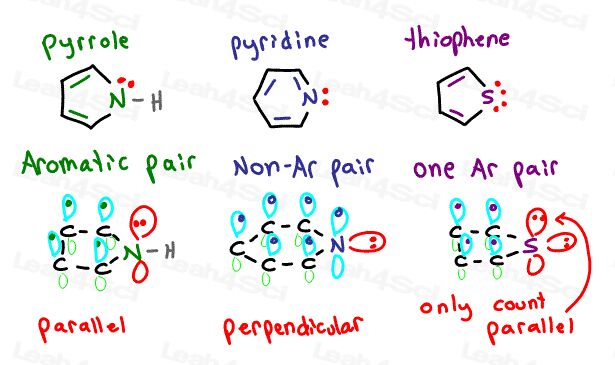
Pay particular attention to the orientation of the lone pair electron orbital.
Pyridine has its p-orbital occupied by pi electrons perpendicular to the resonating aromatic ring.
It’s lone pair sits in a perpendicular orbital TOO FAR from the resonating system thus unable to participate
Pyrrole has a hydrogen atom in the perpendicular sp2 orbital. Hydrogen does not participate.
However, it’s lone electron pair sits in a parallel p orbital. This lines up perfectly with the resonating pi bonds allowing Nitrogen to participate.
Thiophene has 2 different types of orbitals for its lone pair.
One lone pair sits in a perpendicular sp2 orbital, far away from the pi bonds and unable to resonate.
The second lone pair depicted in red sits in a p-orbital parallel to the pi bond system. This allows it to participate in the aromatic resonance.



