 As an organic chemistry student you will find yourself faced with acid/based questions again and again.
As an organic chemistry student you will find yourself faced with acid/based questions again and again.
From categorizing molecules to ranking their strength without pKa or pKb values. This isn't terrible if/when you learn it correctly, however, your professor will assume you already know this.
So s/he won’t teach it.
Why?
Because you’re expected to remember this from back in general chemistry.
Instead your professor will fly through the material and expect you to keep up.
But this won't help with the acid/base classifications.
Simply memorizing definitions without true understanding won't make it any easier. Especially when faced with a molecule that appears to fit more than one acid/base definition.
So let's take a look at acid/base definitions in a more logical way.
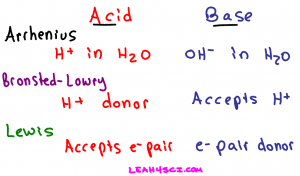
Categories of Acids and Bases
 Before we dive in, be sure to download my FREE acid/base cheat sheet to follow along now and take with you to study on the go – Organic Chemistry Acid Base Cheat Sheet
Before we dive in, be sure to download my FREE acid/base cheat sheet to follow along now and take with you to study on the go – Organic Chemistry Acid Base Cheat Sheet
Acids and bases will fall under one or more of the following three categories:
- Arrhenius acids/bases
- Bronsted-Lowry acids/bases
- Lewis acids/bases
The key here is to recognize that while each classification has a specific definition, any given molecule can fall into more than one category, some into all 3. Again, something we'll look at later in this article.
Arrhenius Acid
An Arrhenius acid is a molecule that when dissolved in water will donate an H+ in solution. Simply put, a proton donor.
The trick to recognizing an Arrhenius acid is to look for a molecule that starts with an H, and typically contains an oxygen or halogen.
Common examples of Arrhenius acids include:
- Hydrochloric Acid – HCl
- Nitric Acid – HNO3
- Sulfuric Acid – H2SO4
- Acetic Acid – HCH3CO2
- and so many more…
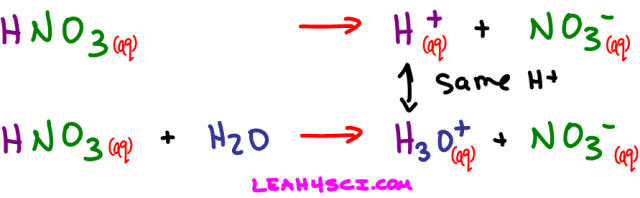
An acid dissociating in water does not form a free-floating proton. Instead one of the water molecules in solution will grab the H+ yielding a hydronium or H3O+ ion. Here's what happens when nitric acid dissociates in water.
Arrhenius Base
An Arrhenius base is a molecule that when dissolved in water will break down to yield an OH- or hydroxide in solution. To recognize the Arrhenius base look for a molecule ending in OH, but not following CHx which refers to an alcohol.
Arrhenius base examples include:
- Sodium hydroxide – NaOH
- Potassium hydroxide – KOH
- Magnesium hydroxide – Mg(OH)2
- and so many more…
But what if the acid/base is not dissolved in water?
The Arrhenius definition for acids and bases only refers to compounds dissolved in water. Does this mean that acids and bases cannot exist out of water? Not quite, that's where the Bronsted-Lowry definition comes in.
Bronsted-Lowry Acid
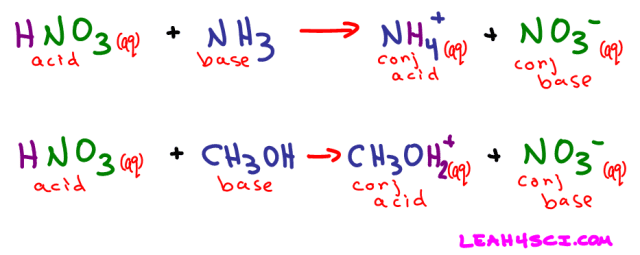
A Bronsted-Lowry acid, like an Arrhenius acid, is a compound that breaks down to give an H+ in solution. The only difference is that the solution does not have to be water. We can still refer to the exact same acids as listed for the Arrhenius acid examples, but this time we'll change the solvent to ammonia, alcohol, or anything else.
We saw what happens when nitric acid (HNO3) dissolves in water. Now let's see what happens when it dissolves in ammonia (NH3) or even methanol (CH3OH)
Nitric acid still dissolved to yield an H+ and NO3-, but this time it was NH3 and not water that picked up the free-floating proton.
Bronsted-Lowry Base
This is where we start to see the difference between the Bronsted-Lowry and Arrhenius definitions. While the Arrhenius base referred specifically to the hydroxide (OH-) ion, the Bronsted-Lowry base refers to any atom or ion capable of accepting or bonding to a free proton in solution.
Referring back to the HNO3 + NH3 reaction above, when ammonia picks up the free H+ it acts as a proton-acceptor. NH3 is the Bronsted-Lowry base in this example.
Additional examples include:
- Methanol – CH3OH
- Formaldehyde – H2CO
- And even water – H2O
Lewis Acids and bases
The Lewis definition for acids and bases is the most extreme because it's not dealing with protons specifically. Instead the Lewis definition deals with the movement of electrons.
Lewis Acid/Base Mnemonic
Think of Lewis as ‘lectrons'
Lewis Acid
A Lewis acid refers to an atom or molecule that accepts an electron pair. Think back to your ‘pushing arrows' for orgo mechanisms. Every time you draw an arrow representing the movement of electrons, the atom getting attacked or accepting those electrons is the Lewis acid in that reaction.
Common Lewis Acid Examples in Organic Chemistry
- Borane – BH3 (hydroboration reaction)
- Aluminum Chloride – AlCl3 (electrophilic aromatic substitution reaction)
- Iron (III) Bromide – FeBr3 (electrophilic aromatic substitution reaction)
- and our good friend H+ (keep reading)
The drawing below is part of the EAS Aromatic Halogenation reaction which you'll see in late Orgo 1 or Orgo 2. Notice how the Fe gets attacked by a lone pair of electrons. By accepting those electrons Fe acts as a Lewis acid
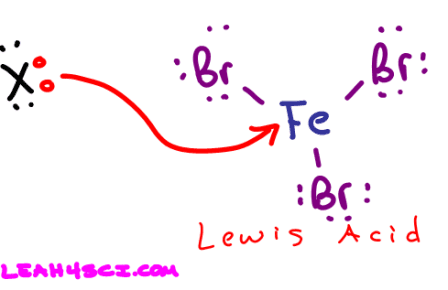
Lewis Base
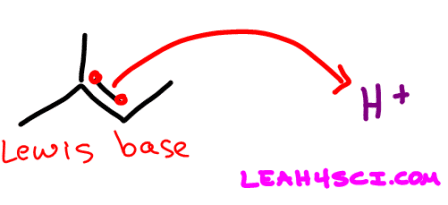
Since the Lewis definition has to do with the transfer of electrons, you can guess by now that a Lewis Base is an electron pair donor. Once again think back to your reaction mechanisms. The molecule using its electrons to attack another atom is an electron pair donor and a Lewis Base.
Here is the first step in acid catalyzed hydration. The pi bond attacking H+ makes the alkene a Lewis Base.
What If It Fits More Than 1 Category?
After learning the individual acid/base definitions you're likely confused and overwhelmed.
So much information and so very similar!
And what happens if a molecule appears to fit more than one category?
For example:
Is OH- an Arrhenius Base? Or perhaps its a Bronsted-Lowry or even Lewis Base?
The answer is potentially all 3!
The definitions above evolved slowly as scientists were starting to understand more and more details about chemical reactions. The first is a basic (no pun intended) definition, but the last 2 simply expand to fit more complex solvents and situations.
Take a look at the reaction below where the hydroxide ion attacks a proton on hydronium.
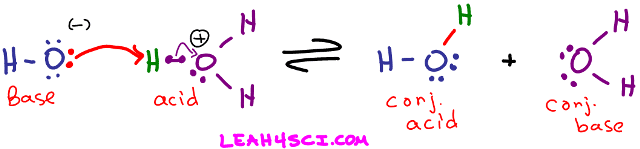
Arrhenius Acid Definition: Hydronium breaks up to yield an H+ in solution.
Arrhenius Base Definition: Hydroxide is an OH- dissolved in water.
Bronsted-Lowry Acid Definition: Hydronium is an H+ donor regardless of solution
Bronsted-Lowry Base Definition: Hydroxide attacks and accepts the H+ from hydronium.
Lewis Acid Definition: The H+ on Hydronium accepts the attacking electron pair to form a bond.
Lewis Base Definition: Hydroxide donates its electron pair to form a bond between itself and H+
Now that you understand the similarities/difference between Arrhenius, Bronsted-Lowry, and Lewis acids and bases, how do you tell which is the stronger or weaker acid? Which molecule is more reactive? Which side of a reaction will be favored at equilibrium?
Learn this and more in my acids and bases tutorial video series:
In conclusion:
As an organic chemistry student you will be required to recognize and classify 3 different types of acids and bases. Arrhenius, Bronsted-Lowry, and Lewis. While the technical definitions vary, once you get the logic behind their definitions you'll be able to quickly and easily identify the different types of acids and bases.
Want to test your understanding of acids and bases? Click to try the FREE Acid/Base practice quiz.



Wtf nigga this is better then my damn teacher
Please can u explain in just two sentences each
Thanks alot
Thanks a lot. Can give my exam easily now.
Thanks girl…best explanations!!??
Thank u sooooooo much!
Thank you
Thanks
Waow clear and understandable definitions and solution, tanks for the great work done.
Tnk u so much
U r a heaven sent
Hello. Very helpful article. Thanks.
knowledgeable and interesting article. Can you plz provide the chemical equation of it,?
wow, very nice article about the Arrhenius base. Really knowledgeable and interesting. Keep posting like this. Thanks
Is there any acic or asic acid is found?.
Thanks
Love You so much for u r help
That was very informative
Thanks
ALCL3 PKA VALUE?
Thank you. I was stuck with a homework question but I found the answer here. Your explanation is very nice.
It helped me a lot
Thank u
Definitely clarifies. I really wish to instructors would go back and apply the definitions when dealing with EAS and nucleophilic attack. So many contextless lectures.
You’re Awesome Teach!!!
Nice work, clear and easy to understand. I will like to learn more about the 3d series of transition elements.
Thank you so much! This makes so much more sense than my chem lecture ahh
Thank you
I love how you compared all three in one place- so helpful!
Something different and understandable………thnx
Tanx lea for ur wanderful explations
even though you are not a teacher but would have been a gud teacher. Keep it up
Amazing!!!! I love Chemistry already. Thanks for the infos. I don’t want to be a laughing stock when I report.
I hav a doubt plz help in the reaction NH3 + H2O goes to( NH4+) + OH- h20 acts as a proton donar as well as OH- donar so according to bronsted lowry defination h20 is acid and according to arrehenius it is base , i m confused plz help
Is HCl as a whole Lewis acid? Or is it H+ in it?
You’re welcome, Sera!
Thanks a lot. ..
You’re welcome, Tamim!
Thank you so much, I am definitely going to ace the test tomorrow!
Thanks your explanation you are so lovely
Love girl
Great one, all is clear dear! ..thank you
You’re welcome Alhassan
Thank you
You’re welcome Kamille
Thank for your good explaexplanations
You’re welcome!!
Thank you so much… Nice n clear explanation! Do you teach physical chemistry?
Thank you and no, not at this time
nyc explanation
Thanks Haseeb
Thank you very much! Your explanation is so clear, the examples are relevant and straight to the point. I cannot thank you enough
You’re very welcome Jonas! Glad to help
Hi I am trying to study for p cats I know there is a short cut to all chemistry.and math I just need to know what it is. I have taken this test p cats before and I need to score much higher there is to much competiton for p cats to put this on the internet. I could use some help, I have taken chemistry 1,2 and organic I and 2. I could not score higher than c..My professor said I was to old to be a pharmacist and she would not give me higher than c in all science chem l and 2 org 1 and 2. I think that was a terrible thing to say to any one. I will not give up and I am very passionate about pharmacy. can you help. I am a single parent 3 kids 2 in college I in high school. tutoring is to expensive at this time I need to raise my science gpa and take the pcats over. I spoke to a pharmacy school and that was the suggestion they gave me. aaaballiet@gmail.com. do you think you can help thank you , carrie .
you are awsome Leah.
Thank you Pankaj
clear explanation, and would like to learn more
Thank you Banda. When you say ‘learn more’ what specifically are you referring to?