 When it comes to organic chemistry reactions,
When it comes to organic chemistry reactions,
many students focus on learning the EXACT steps by memorizing the SPECIFIC molecules presented in their books and lecture slides.
Then, when something looks even slightly different on the exam, they panic!
One such scenario: replacing water with alcohol in a reaction sequence – either as the attacking molecule or as the solvent.
For example:
Acid catalyzed hydration using an alcohol solvent instead of water.
Or Oxymercuration using alcohol instead of water for the Alkoxymercuration reaction.
How do you quickly mentally swap the water reaction into an alcohol?
How do you easily complete the new reaction?
Enter the Backpack Trick
Forget organic chemistry and bear with me for a moment.
No matter what you do, You are You.
Right?
You may change your hair,
You may change your clothes,
But You are STILL You.
Say you’re walking down the street,
And your friend recognizes you.
They recognize you because you are you.
Now imagine that you’re walking down the street wearing a heavy backpack.
Thanks to a big exam tomorrow you have about 20 pounds of textbook, notes, and more weighing you down.
Are you still you?
Absolutely!
Are you any different?
Perhaps you’re walking differently to compensate for the weight.
Perhaps you have trouble getting onto the crowded morning train due to the bulk of your backpack.
Perhaps the other commuters can’t crowd you as tightly due to the bulk of your backpack.
(I used to purposely wear my backpack in the front on a crowded bus/train preventing people from crowding my breathing space).
But you are still you,
You just happen to be carrying a whole lot more than you normally do.
Hang on to that thought for a minute.
The reactivity of water:
What makes water such an ideal solvent for reactions?
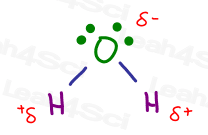
The water molecule H2O features two hydrogen atoms covalently bound to oxygen.
But this is not a ‘balanced’ covalent bond.
Oxygen is highly electronegative and pulls on the electrons between itself and hydrogen. This concentrates negativity around the oxygen atom, leaving both hydrogen atoms partially exposed with a partial positive charge.
To really understand this we need just ONE oxygen and ONE hydrogen.
In most reactions, the oxygen is ultimately de-protonated so that just one hydrogen is removed.
So let’s assume the other hydrogen is just extra baggage,
… a backpack.

If we call that extra hydrogen a backpack and turn it into a circle, does the reaction still proceed as expected?

Yup, nothing changed. It’s just the OH reacting with a backpack.
Now what if I turn that backpack into something else,
For example an ANY alkyl group represented by the letter R.
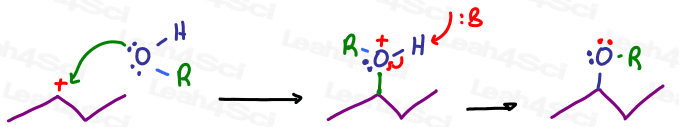
Same mechanism, but since the product has an OR instead of an OH the product is an ether instead of an alcohol.
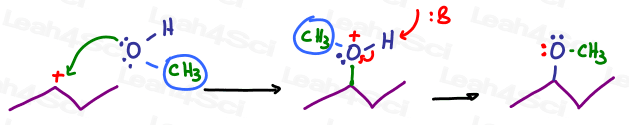
Let’s try another backpack using a methyl group:
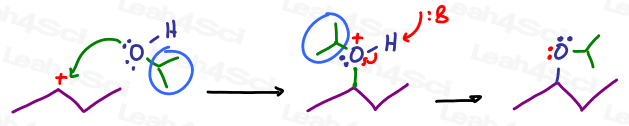
And an isopropyl group:
Do you see how oxygen still reacts the same way?
Oxygen's electrons still attack the carbocation, and the one hydrogen is still removed in the final deprotonation step. This happens regardless of the other group or backpack attached to the carbon atom.
Backpack Shortcut For Reactions
If this makes sense when the mechanism is drawn out,
how can you apply the backpack trick when working quickly through reactions?
As with any trick or shortcut:
FIRST learn and understand the logic BEHIND the trick or shortcut.
Then study the fast and simple method to save you time.
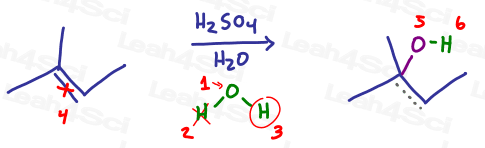
Let’s follow the logic of this reaction. In each scenario above:
- Oxygen attaches to the more substituted carbon of the initial pi bond
- The hydrogen atom is removed via a final proton transfer step
- The backpack is attached to oxygen
Using this logic, here’s the shortcut:
(Match the numbers to the reaction shortcut steps below.)
- Identify your oxygen atom carrying a backpack
- Cross out the hydrogen atom (lost to proton transfer)
- Circle the backpack
- Now for the reaction: Break the pi bond
- Attach an oxygen atom to the more substituted carbon
- Draw the backpack coming off the oxygen atom
That’s it, that’s your final product.
Let’s see how this works for acid catalyzed hydration with water:
Now the same reaction with an alcohol:
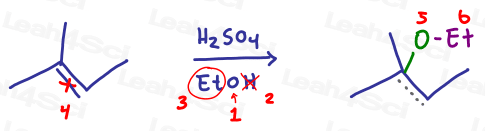
Let’s try this with oxymercuration-demercuration:
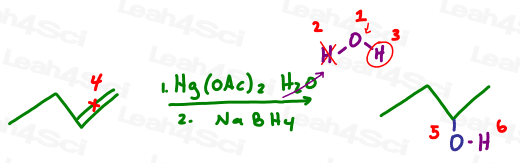
And now let’s try alkoxymercuration which is the same reaction as above but with alcohol, ie: oxygen carrying a carbon chain backpack.
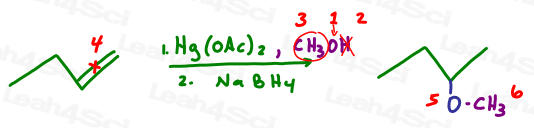
This tutorial is just the first step to learning and understand organic chemistry concepts and reactions. Don’t let the semester take you by surprise forcing you to play catch-up. Click below to download EVERY Leah4sci Orgo Cheat Sheet in a single full color PDF download. You’ll also get access to tutorials, study tips and tricks, and Upcoming LIVE Workshop announcements!


