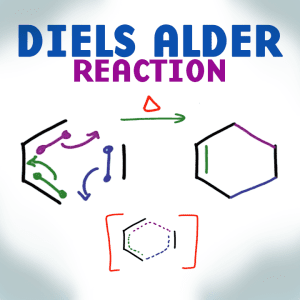
 When it comes to complex orgo reactions, Diels Alder is one of my favorite, perhaps second to the Aldol/Claisen condensations. This reaction is one that appears tricky at first, especially when faced with heavily substituted or cyclic reactants. However, if you follow my pattern and apply the tricks I teach you here, Diels Alder will be a source of ‘free points' for you any time it shows up on your exam.
When it comes to complex orgo reactions, Diels Alder is one of my favorite, perhaps second to the Aldol/Claisen condensations. This reaction is one that appears tricky at first, especially when faced with heavily substituted or cyclic reactants. However, if you follow my pattern and apply the tricks I teach you here, Diels Alder will be a source of ‘free points' for you any time it shows up on your exam.
Reaction Guide and Practice Problems to follow (Coming soon)
Need to find HOMO and LUMO molecular orbitals for molecules in these reactions? Visit my Molecular Orbital Theory page.
Video 1 – Diels Alder Reaction, Mechanism, and Product Trick
Video 1 in the DA series helps you understand the cycloaddition reaction mechanism and intermediate. This video also teaches you my trick for quickly identifying DA products from any given starting diene and dienophile, and the reverse trick for finding reactions from the cyclohexene product.
Video 2 – Diels Alder for Cyclo Reactants and Bicyclo products
 Video 2 shows you how to apply the tricks from video 1 to cyclic starting molecules. You'll also learn how to find the starting diene/dieonophile from a bicyclic product.
Video 2 shows you how to apply the tricks from video 1 to cyclic starting molecules. You'll also learn how to find the starting diene/dieonophile from a bicyclic product.
Video 3 – Diels Alder Reaction Stereochemistry and Endo vs Exo Products
Video 3 gives you tips on showing the stereochemistry for Diels Alder products when substituents are present on both the diene and dienophile including stereochemistry for a bicyclic product. You’ll also learn to distinguish between endo and exo products for dienophiles with both cis and trans configurations.



