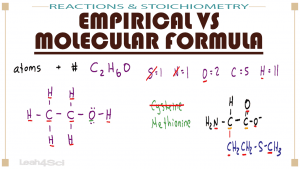
 Navigating through empirical formulas versus molecular formulas will help you in conversions, unknowns, and so many exam problems.
Navigating through empirical formulas versus molecular formulas will help you in conversions, unknowns, and so many exam problems.
This video explains the definitions, similarities, and differences between an atom's empirical formula and its molecular formula.
You will also see when the empirical and molecular formulas are the same, and a percent by mass example!
(Watch on YouTube: Empirical vs Molecular. Click cc for transcription.)
Links & Resources Mentioned In This Video:
<– Watch Previous Video: Calculating Molecular Weight and Formula Weight
–> Watch Next Video: Molarity, Molality, and Molar Mass


