 Alkene Reactions Series: Video 4
Alkene Reactions Series: Video 4
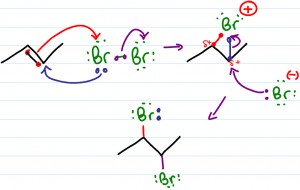
Halogenation of alkenes is the reaction in which a double bond is broken and replaced by a vicinal dihalide – 2 halogen atoms added to neighboring carbons. This reaction follows a pattern of anti addition.
The goal of this video is to help you understand rather than memorize concepts related to the halogenation mechanism. This video addresses dihalide polarization, bromonium/chloronium bridge formation, addition to cyclic and asymmetrical starting alkenes.
(Watch on YouTube: Halogenation. Click cc on the bottom right for video transcription.)
<– Watch Previous Video: Hydrohalogenation of Alkenes
–> Watch Next Video: Halohydrin formation
This is Video 4 in the Alkene Reaction Mechanisms Video Series. Click HERE for the entire series.
Ready to test your skills? Try the Alkene Reactions Practice Quiz after watching the series!


Why does the bromine attack back with its elections? I don’t see what the driving force for that would be.
Once the pi bond attacks the bromine, doesn’t that make a bond?
Hi, i would like to know why the chlorine was not on wedges or dashes in the last example.
Why does chlorine not form a bridge like bromine?
Hi Leah, since the bromine in the bridge has a positive formal charge and the other bromine is negative. Why is the negative bromine attracted to the partially positive carbon instead of the other positive bromine?
Thanks
Do you have any videos on Radical Rxns, NBS, Allylic carbon, how to calculate delta H for propagation steps? That is what I am doing now in my Orgo II class.
I cover some of these topics in the Organic Chemistry Study Hall. Join here: https://leah4sci.com/join
Hi Leah, I think you are doing a fantastic job. I think you can add a DONATE button so that people can donate to you. It is another way people can say thank you!!
Thanks for the suggestion Munyaradzi! The best way to donate is
1- sign up for my services or study hall
2- tell your friends about my free resources and perhaps they will sign up
In the last example, propene reacting with Cl2, do we still worry about stereochemistry?
Only for the central carbon which WILL be chiral but racemic
Hi all. The description of resonance in the bromonium ion intermediate is rather misleading. The contributing structures have no independent existence. There is no “back and forth” between them. The actual structure is a sort of average of them, and always contains two bonds to the bromine atom.
You are correct. The video is taught to help understand the partial positive charge (unless I made a mistake which sometimes happens)
Hi Leah, I’m confused as to why the bromine in the bromine bridge has a positive charge, when it has three lone pairs, and two bonding electrons. Shouldn’t it have a negative charge? Or maybe should have only two lone pairs?
Actually the bromine in the bridge has two lone pairs and two bonding pairs. Since bromine is in group 7, the calculation is 7 – 2×2 -2×1 = +1.
Yup that was an error I caught after posting. YouTube doesn’t let me correct live videos and I won’t delete it just for that
hello, so if the bromine in the bridge has two lone pairs and two bonding pairs, where are the pair electrons we started with gone to??
thank you very much for those videos !
Idan: The bonding pair is the former lone pair